Are you looking for Haloalkanes and Haloarenes Handwritten Notes for the Class 12 Board Exam with Downloadable Pdf or Ppt? Then you are in the right place. This post is a complete guide about Haloalkanes and Haloarenes Handwritten Notes.
Haloalkanes and Haloarenes Handwritten Notes: A Complete Guide
Are you struggling with organic chemistry? Haloalkanes and haloarenes are important concepts to understand, yet they can be challenging.
Imagine having handwritten notes that make everything easier for you. These notes will simplify your complex concepts into simple steps, making study sessions less stressful and more productive.
Whether you are studying for an exam or want a better understanding of the content, these notes provide clear, simple explanations that will help you learn faster.
No more feeling lost in a sea of information—just clear advice to make organic chemistry easier and more enjoyable.
Are you ready to master Haloalkanes and Haloarenes? Just make these handwritten notes your primary reference!
What are Haloalkanes?
Haloalkanes, also known as alkyl halides, are compounds in which one or more hydrogen atoms in an alkane are replaced with a halogen (such as fluorine, chlorine, bromine, or iodine).
They are divided according to the carbon atom to which the halogen is attached:
- primary (1°) haloalkanes, or, mono haloalkane
- secondary (2°) haloalkanes, or, dihaloalkane, and
- tertiary (3°) haloalkanes, or, trihaloalkane.
This classification is significant since it determines how certain substances react in various chemical reactions.
One of the most important reactions involving haloalkanes is nucleophilic substitution, which involves replacing the halogen atom with a nucleophile. Understanding this reaction is critical since it serves as the foundation for a variety of other organic chemical processes.
Moreover, haloalkanes undergo elimination processes in which the halogen and a hydrogen atom are eliminated to yield an alkene. These reactions may appear confusing at first, but once broken down into steps, they become lot more understandable.
To properly understand haloalkanes, learn the patterns and trends rather than remembering each reaction separately. Because of the lower steric barrier, primary haloalkanes are more likely to undergo nucleophilic substitution than tertiary ones.
Tertiary haloalkanes, on the other hand, are more likely to be eliminated because they give rise to more stable alkenes.
Haloalkanes may appear to be a difficult topic, but with the correct resources, they’re actually easy-to-understand.
The idea is to break down the knowledge into reasonable portions and concentrate on figuring out the fundamental principles rather than being weighed down in the information.
By taking precise and straightforward notes, you may make this difficult material simple and even entertaining to learn.
Remember that organic chemistry is a field in which everything is interrelated. Once you understand haloalkanes, many other things will make more sense.
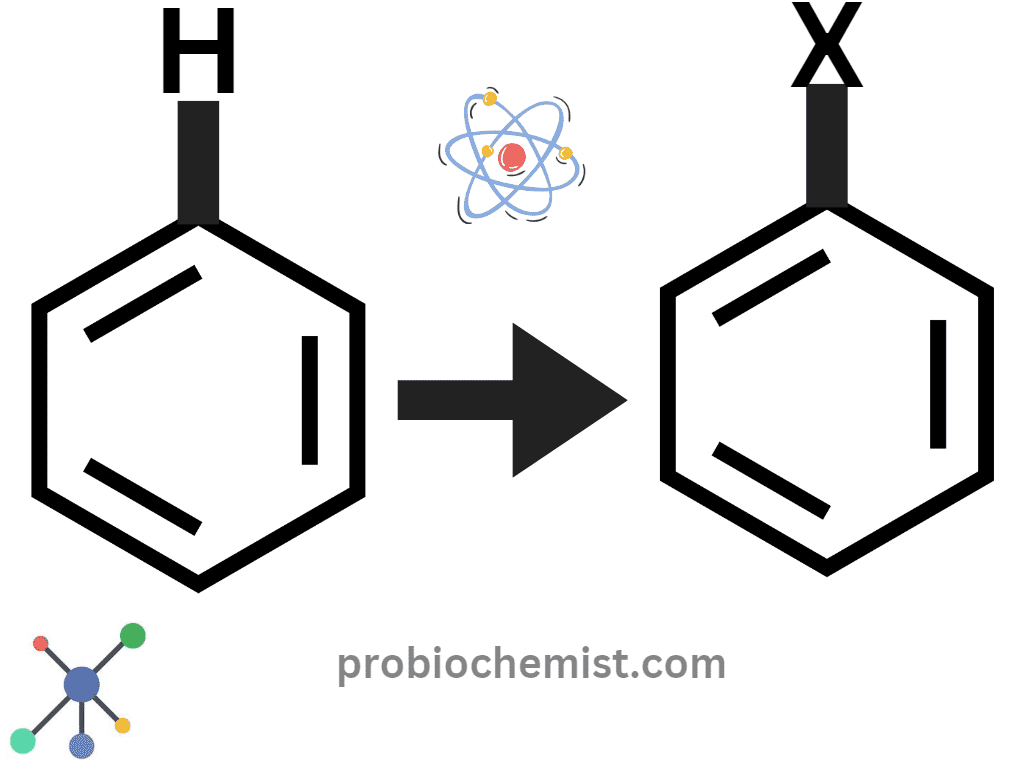
What are Haloarenes?
Haloarenes which are known as aryl halides. These are compounds that include a halogen bonded to an aromatic ring, such as benzene.
Haloarenes, unlike haloalkanes, are less liable to nucleophilic substitution due to the aromatic ring’s stabilizing effect.
Electrophilic substitution is an important reaction to study because it occurs when an electrophile replaces a hydrogen atom on the ring.
Understanding why replacements happen at the ortho, meta, or para locations is critical. As an example, in chlorobenzene, chlorine leads substituents to the ortho and para locations.
Focusing on these fundamental topics will make your study of haloarenes easier to handle and understandable.
Free Download Pdf: Haloalkanes and Haloarenes Handwritten Notes for Class 12
Your Haloalkanes and Haloarenes Handwritten Notes’ PDF is Available here. Please click the button below to download the handwritten notes of Haloalkanes and Haloarenes for Class 12.
Downloadable Haloalkanes and Haloarenes Notes for Organic Chemistry
Grab our Haloalkanes and Haloarenes Handwritten Notes to make studying easier. These notes break down tricky concepts into simple parts, making it easier for you to understand and succeed in your exams.
You may also like:
Structure of Atom Class 11 PPT – Class 10, Class 9
50 Examples Of Balanced Chemical Equations With Answers PDF
Positive Deviation From RAOULT’S LAW – Explaination – Solution
Essential Haloalkanes and Haloarenes Notes for Chemistry Students
Classification of Haloalkanes
Haloalkanes are classified according to the carbon atom to which the halogen is bonded.
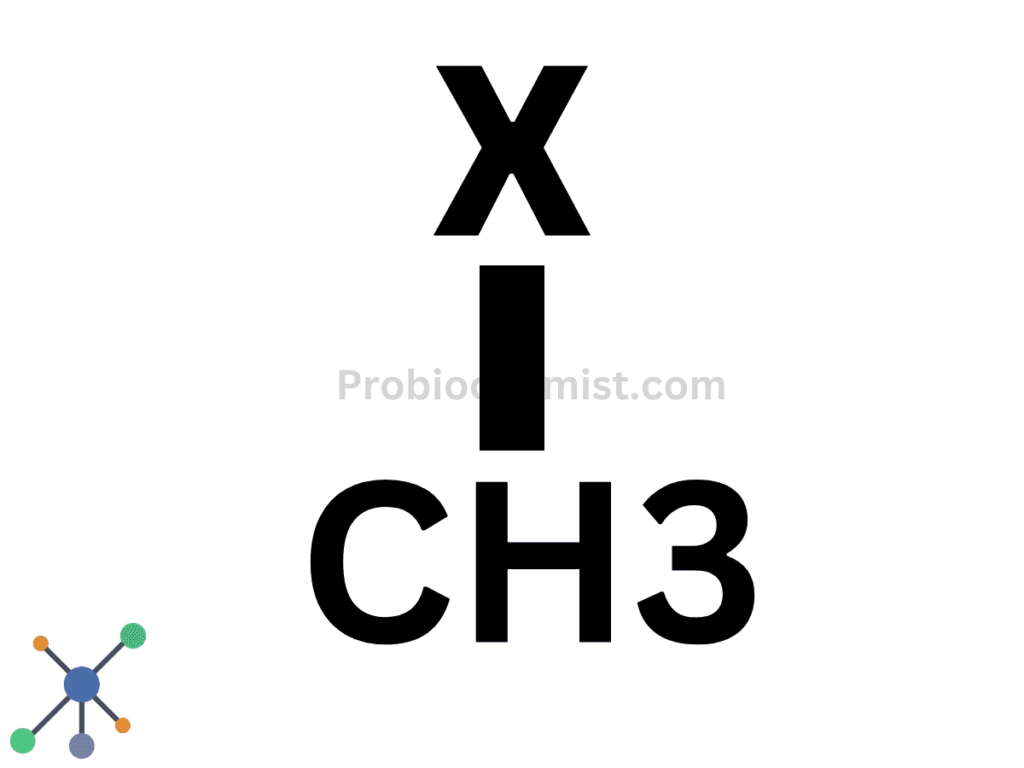
Primary Haloalkanes or 1° Haloalkanes or Monohaloalkane
The halogen is linked to a single carbon atom.
Secondary Haloalkanes or 2° Haloalkanes or dihaloalkane
Secondary Haloalkanes or 2° Haloalkanes are compounds in which the halogen is linked to a carbon atom connected to two other carbon atoms.
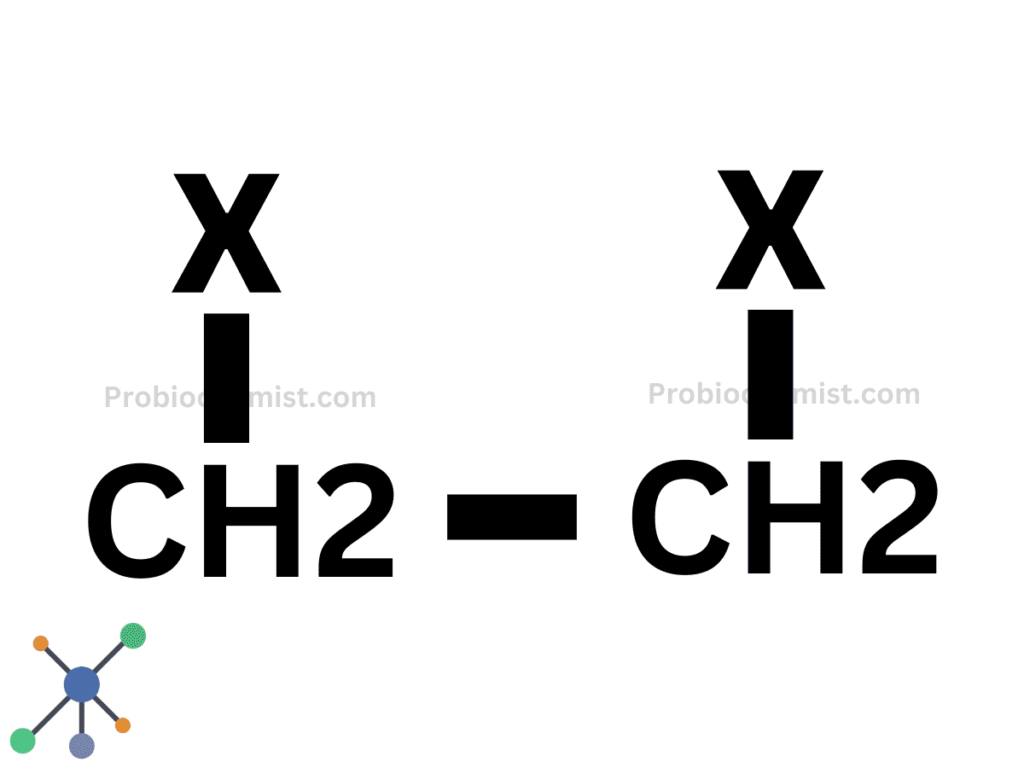
Tertiary Haloalkanes or 3° Haloalkanes or trihaloalkane
Tertiary Haloalkanes or 3° Haloalkanes are compounds in which the halogen is linked to a carbon atom connected to three other carbon atoms.
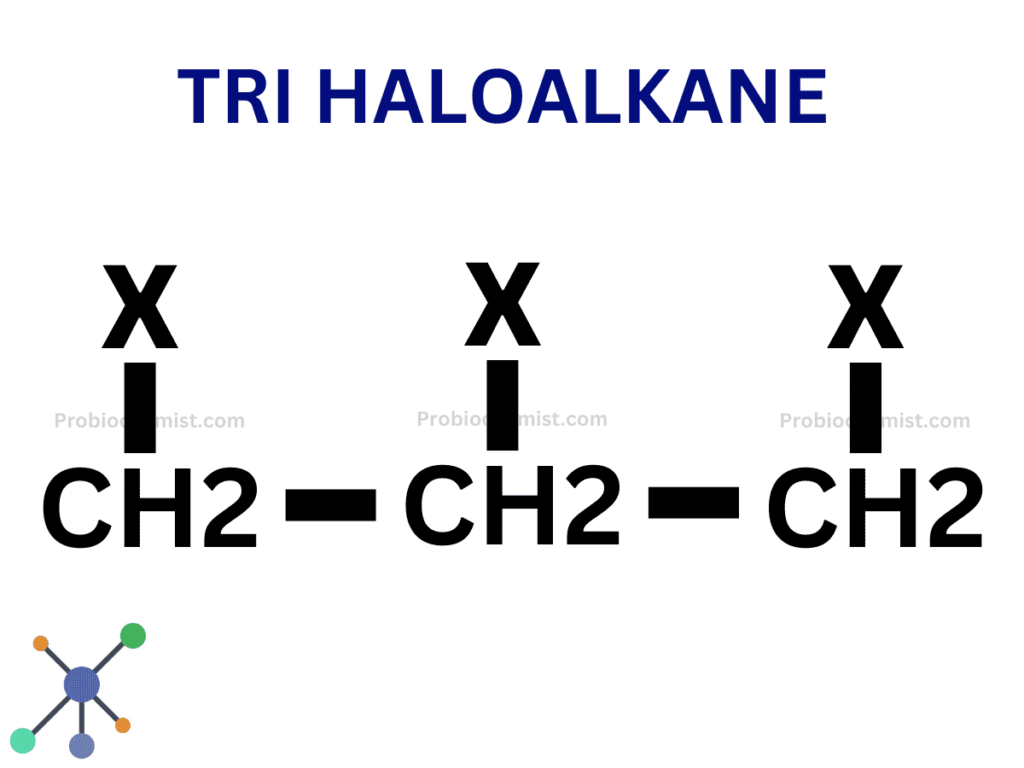
This classification impacts the reactivity of the haloalkane, particularly in substitution and elimination processes.
Reactions of Haloalkanes
Elimination Reaction
Elimination reactions remove the halogen and hydrogen atoms from a haloalkane, resulting in a formation of a substance called alkene.
This reaction is more prevalent in secondary and tertiary haloalkanes. Elimination process follows 2 main mechanisms. Examples of elimination reactions include:
E1 Mechanism:
It is a two-step mechanism for forming a carbocation intermediate.
E2 Mechanism:
It is a one-step mechanism in which halogen and hydrogen atoms are eliminated simultaneously.
Nucleophilic Substitution Reaction
Nucleophilic substitution involves replacing the halogen atom in a haloalkane with a nucleophile, which gives an electron pair.
This reaction occurs more frequently in primary haloalkanes due to their reduced steric barrier. The nucleophilic substitution mostly follows 2 mechanisms, The most frequent nucleophilic substitutions are:
SN1 Mechanism:
A two-step process in which the halogen exits first, resulting in a carbocation intermediate.
SN2 Mechanism:
A one-step process in which the nucleophile approaches the carbon atom while the halogen leaves.
Applications of Haloalkanes: A Class 12 Guide
Haloalkanes are frequently employed in several sectors. They are essential in the manufacture of solvents, refrigerants, and medicines.
As for example, chloroform and carbon tetrachloride are typical solvents, whereas halothane is an inhalational anesthetic used in surgeries.
Structure of Haloarenes
Resonance stabilizes the aromatic ring, which is the main characteristic of haloarenes.
Different reactivity patterns result from this resonance, which strengthens the carbon-halogen link in haloarenes relative to haloalkanes. While they take part in electrophilic substitution reactions, haloarenes are less reactive in nucleophilic substitution reactions.
Classification of of Haloarenes
The amount of halogen atoms present in haloarenes can be used to classify them as follows:
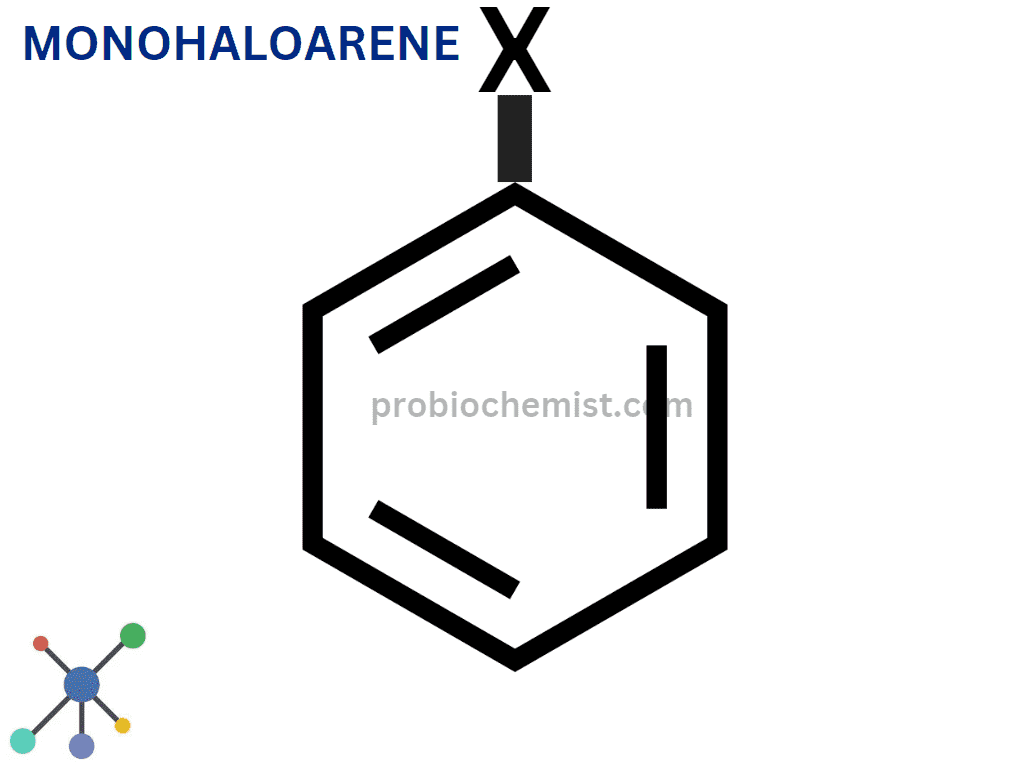
Monohaloarenes
One halogen atom is present in monohaloarenes. As for example, chlorobenzene (C6H5Cl) is an example of a compound.
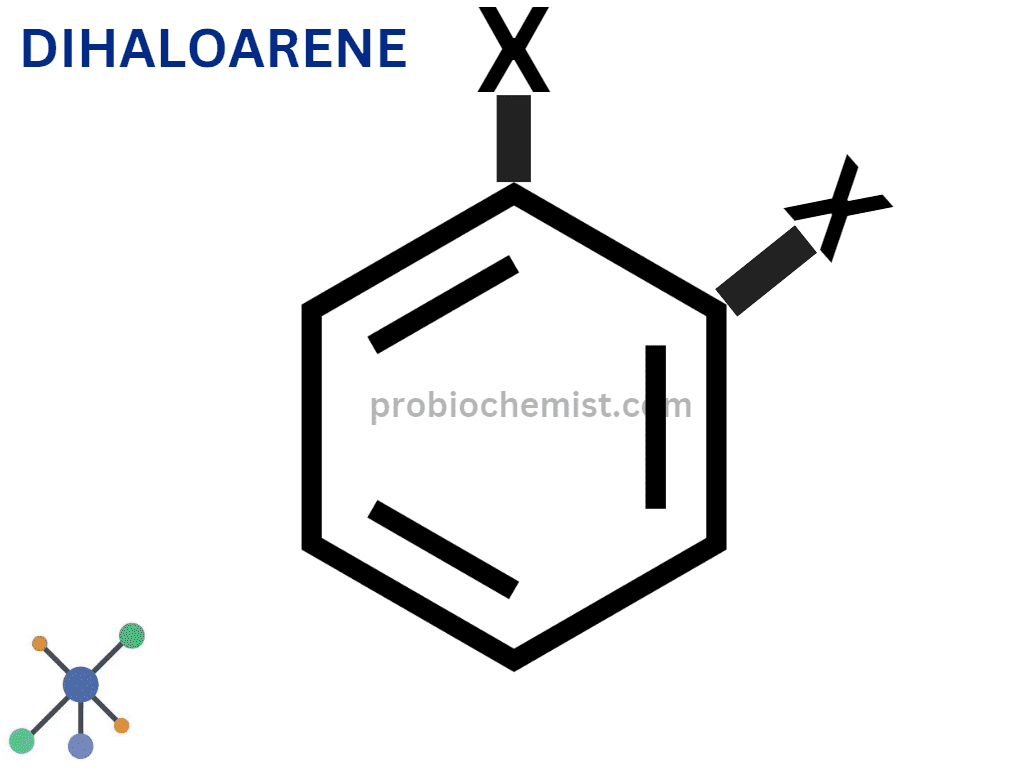
Dihaloarenes
Dihaloarenes: Have two atoms of halogen in them. As for an example, consider dichlorobenzene (C6H4Cl2).
Polyhaloarenes
More than two halogen atoms can be found in polyhaloarenes.
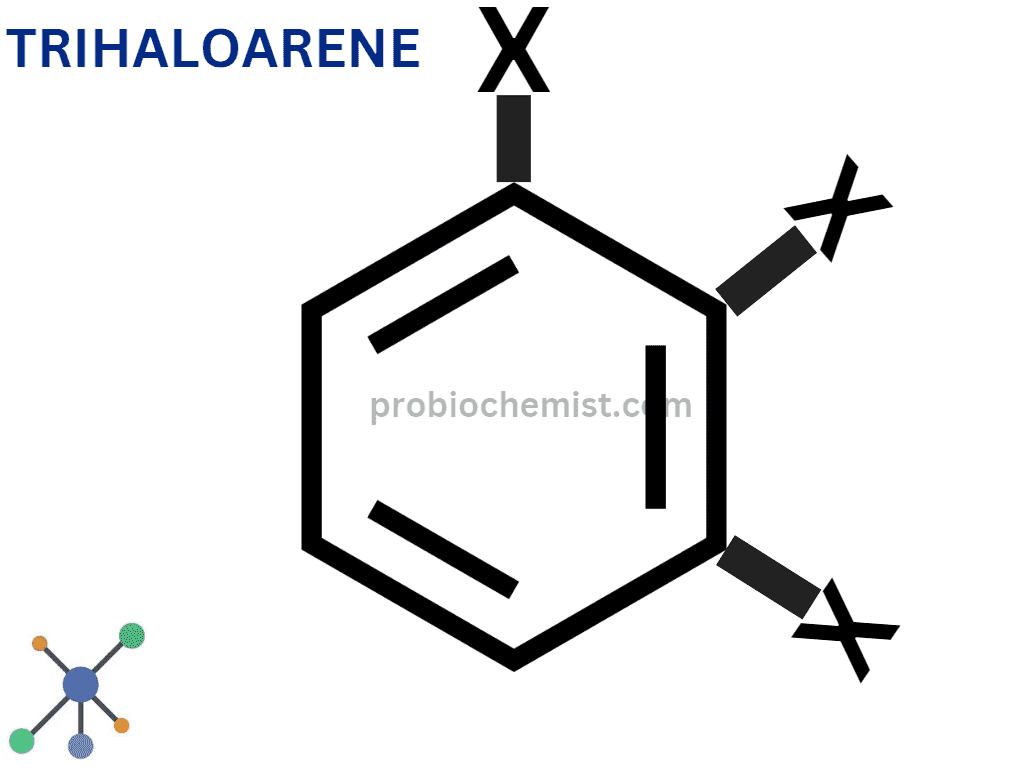
Example: trichlorobenzene (C6H3Cl3) is an example of a compound.
Reactions of Haloarenes
Electrophilic Substitution: In this process, an aromatic ring hydrogen atom is swapped out for an electrophile, which is an atom or molecule that can receive an electron pair.
The halogen atom that is already bonded to the ring affects the location where the substitution takes place. Since halogens are ortho or para-directing groups, fresh groups are most likely to attach to the ring’s locations opposite (para) or next to (ortho) the halogen.
Typical electrophilic substitution processes consists of:
Nitration
The addition of a nitro group (NO₂) to an aromatic ring is known as nitration.
Halogenation
The process of adding a halogen atom, such as bromine or chlorine, to an aromatic ring is known as halogenation.
Sulfonation
The addition of a sulfonyl group (SO₃H) to the aromatic ring is known as sulfonation.
Applications of Haloarenes
Agrochemicals, medications, and dyes are all made from haloarenes.
As for example, numerous compounds, such as the solvent phenol and the insecticide DDT, are derived from chlorobenzene. In addition to its use in medicine, bromobenzene is synthesised into other chemical molecules.
Top 6 Difference Between Haloalkanes and Haloarenes
| Haloalkanes | Haloarenes | |
| Structure | 1. Haloalkanes are the base from which halogen atoms (F, Cl, Br, I) are substituted for one or more hydrogen atoms to create haloalkanes. There are branches and linear sections in the open carbon chain. | 1. Haloarenes are aromatic compounds (such as benzene) in which one or more hydrogen atoms are swapped out for halogen atoms in the aromatic ring. Planar and stable is the ring structure. |
| C-H Bond | 2. Due to the difference in electronegativity between the halogen and carbon atoms, the carbon-halogen bond in haloalkanes is usually polar. Haloalkanes have sp3 hybridised carbon. | 2. Resonance causes the carbon-halogen link in haloarenes to be less polar. In haloarenes, the halogen engages in resonance with the aromatic ring after the carbon atom undergoes sp2 hybridisation. |
| Reactivity | 3. Due to their greater ability to break the carbon-halogen link, haloalkanes are often more reactive to nucleophilic substitution processes. | 3. Because electrons delocalise over the aromatic ring, strengthening the carbon-halogen link, haloarenes are less reactive to nucleophilic substitution processes. |
| Physical Properties | 4. Haloalkanes usually shows distinct physical characteristics in contrast to haloarenes. For instance, because of the halogen, they often have greater densities and boiling temperatures than their parent alkanes. | 4. Because the aromatic ring adds extra interactions between molecules, haloarenes typically have greater boiling temperatures than haloalkanes with equal molecular weights. |
| Mechanism of Reactions | 5. Haloalkanes, By means of processes like SN1, SN2, E1, and E2, undergo nucleophilic substitution and elimination reactions. The kind of haloalkane (primary, secondary, or tertiary) and the characteristics of the nucleophile or base affect the reactivity and process. | 5. Haloarenes usually undergo aromatic substitution by electrophilia as opposed to nucleophilia. Because of its ability to withhold electrons, the halogen can act as an ortho or para director in electrophilic aromatic substitution processes. |
| Impact of Halogen Substitution | 6. The reactivity of substituted haloalkanes varies according to the kind of alkyl group (primary, secondary, or tertiary) and the halogen present. | 6. Because of the halogen’s directing effects, substituted haloarenes exhibit varying reactivity depending on where the halogen is located on the aromatic ring in relation to other substituents. |
Best Haloalkanes and Haloarenes Notes for Board Exams
Comprehensive Guide to the Preparation of Haloalkanes: Get Your Haloalkanes Handwritten Notes
There are various ways to prepare haloalkanes, and each one requires particular conditions and reagents.
Preparation of Haloalkanes by Free Radical Halogenation Method
The process of free radical halogenation of alkanes is one of the easiest ways to prepare haloalkanes. In this reaction, halogen atoms — typically chlorine or bromine — replace the hydrogen atoms in an alkane. Heat or UV radiation both start the process by producing free radicals, which accelerate the reaction.
CH4+Cl2 (In presence of sunlight)→ CH3Cl+HCl
Simple in approach this process lacks selectivity and frequently produces a variety of products, particularly when higher alkanes are utilised.
Electrophilic Addition of Hydrogen Halides to Alkenes
Hydrogen halides (HX) added electrophilically to alkenes is another popular technique for creating haloalkanes.
CH2 = CH2 + HBr → CH3CH2Br
The alkene’s double bond is broken in this reaction, causing the hydrogen atom to cling to one carbon atom and the halogen atom to cling to the other.
According to Markovnikov’s rule, this process is extremely regioselective.Halogen links to carbon atoms with a smaller amount of hydrogen than hydrogen atoms, whereas hydrogen atoms bind to carbon atoms with a greater number of hydrogen atoms.
A vital aspect of organic chemistry is the synthesis of haloalkanes, which can be done in a number of ways, each suitable for a particular kind of substrate and the end product.
Guide to the Preparation of Haloarenes: Get Your Haloalkanes Handwritten Notes
These substances have numerous industrial uses and are essential organic synthesis catalysts. Haloalkanes can be made in a number of ways, each requiring certain steps and chemicals.
Preparation of Haloarenes by Nucleophilic Substitution of Alcohols
Alcohols can also be changed into halides to prepare haloalkanes.
In order to accomplish this, an alcohol is usually reacted with a halogenating agent, such as hydrogen halide (HX), phosphorus tribromide (PBr₃), or thionyl chloride (SOCl₂).
As for an example, ethyl chloride (C₂H₅Cl) is created when ethanol (C₃H₅OH) combines with hydrogen chloride acid (HCl).
CH3CH2OH + HCl → CH3CH2Cl + H2O
Thionyl chloride is especially helpful since it generates easily removable gaseous byproducts (SO₂ and HCl), making the haloalkane’s purification process easier, quicker and simpler.
Electrophilic Addition of Hydrogen Halides to Alkenes
Adding hydrogen halides like Hydrochloric Acid, Hyrogen Bromide, or HI to alkenes is another popular technique to manufacture haloalkanes.
The alkene’s double bond is broken during this reaction, causing the hydrogen atom to remain attached to one carbon atom and the halogen atom to adhere to the other.
CH2 = CH2 + HBr → CH3CH2Br
Bromoethane (C₂H₅Br) is the product of the reaction between ethene (C₂H₄) and hydrogen bromide (HBr).
The hydrogen atom connects to the carbon atom in this reaction in accordance with Markovnikov’s principle, producing a regioselective product.
On the other hand, when peroxides are utilised, especially with HBr, the opposite phenomenon known as anti-Markovnikov addition may happen.
There are several ways to synthesise haloalkanes, and each is appropriate for a particular set of circumstances and intended results.
Chemists are able to produce haloalkanes for a variety of uses by selecting the right procedure.


Conclusion
To summarise, students who want to do well in organic chemistry, especially in Class 12, must learn how to prepare haloalkanes and haloarenes.
Being able to comprehend and apply these ideas will help you succeed academically and provide a solid basis for your future chemical studies. Comprehensive “Haloalkanes and Haloarenes Handwritten Notes” are very helpful, whether you are studying complex subjects or just the fundamentals.
These notes can help clarify and simplify difficult subjects.
Make sure to review your “haloalkanes and haloarenes class 12 handwritten notes” as you get ready for your tests. They might be a dependable study tool because they are designed to efficiently cover the course.
Through regular examination of our “Haloalkanes and Haloarenes Handwritten Notes,” you can make sure that you are ready and feel secure when answering questions in this crucial chapter. Warm regards for your academic pursuits!
To ensure that you fully understand the material, keep editing your “haloalkanes and haloarenes class 12 handwritten notes”; if you do this, you should get great grades. Wishing you good fortune!