This article will provide you with 50 best examples of balanced chemical equations with answers and PDF. These chemical equations are very much helpful for class 7, 8, 9, 10, 11, 12 and graduate students of chemistry.
This stoichiometry exercise is the practice Balancing Chemical Equations and it has an answer key to check your gone through process.
Across all balanced chemical reactions the number of atoms on both sides should be same for any element. This will prevent the volume of matter from being created or destroyed during a process.
So, in this blog post I will go through 50 balanced chemical equations along with the method to balance them and also provide you their answers. So, this guide is for all students kids geeks or any one who wish to learn chemistry.
For beginners, balancing chemical equations can seem a bit challenging. When you read your first complex equation with tons of individual elements and polyatomic ions, it is easy to become intimidated.
Where do you start, how does everything get weighted properly and why the hell is this even important?
This may error in the calculation and misconception of chemical reactions which do not exist but due to unbalanced equations students might take it wrong that kind of product is forming, hence they can get wrong experimental results also.
Try to do an experiment with a unbalanced equation. You might get chemicals in the wrong proportions causing danger in a laboratory.
- 50 Examples Of Balanced Chemical Equations With Answers
- Examples Of Chemical Equations With Answers for Combustion Reactions
- Examples Of Chemical Equations With Answers for Decomposition Reactions
- Examples Of Chemical Equations With Answers in Redox Reactions
- Examples Of Chemical Equations With Answers for Organic Reactions
- How to Balance a Chemical Equation With Example?
- Why Should Chemical Equations Be Balanced?
- What is the Trick to Balance a Chemical Equation?
- Can any Chemical Equation Be Balanced?
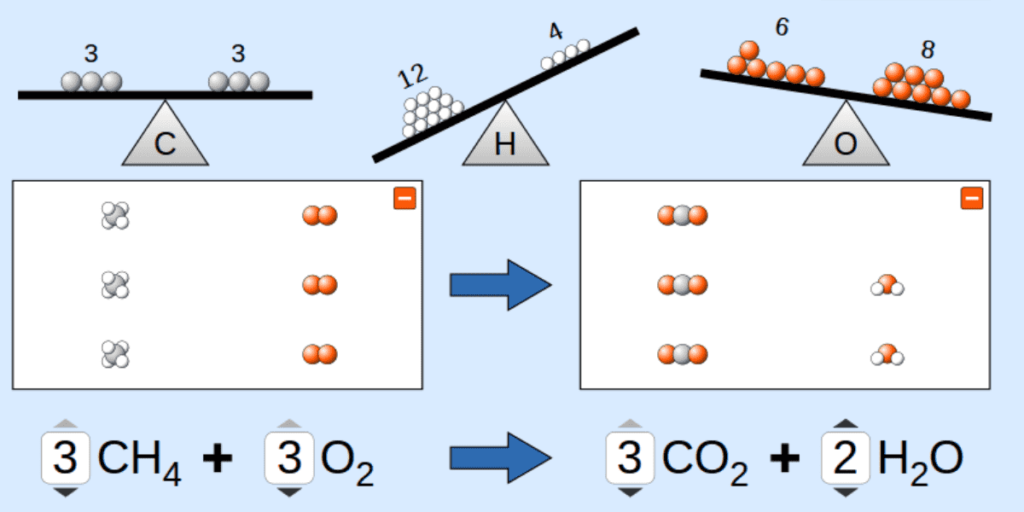
50 Examples Of Balanced Chemical Equations With Answers
The inability to receive the correct answer quickly can be disheartening because this step is so necessary in chemistry studies.
You need practice on isolating variables when balancing equations.
This skill is essential because every reaction in chemistry (from simplest to more complicated reactions) need a balanced equation, and without it, you will face some difficulties while moving ahead in chemistry.
Balancing chemical equation is skill and can be improved by various practice courses.
This Blog post provide you 50 balanced equations for your practice which will help and also provides the answer to those examples. After reading this article, you should feel comfortable of how to approach and solve these equations.
Steps to Balance a Chemical Equations With Answer
- Unbalanced equation: Begin with the proper formulas for each reactant and product.
- Determine the number of each type of atom. How many of each atom is in this equation on either side
- Adjust the atoms individually Elements that remain as reactant and product
- Balance the elements with coefficients. Put numbers before the chemical formulas to balance the amount of atoms.
- Check your work. Make sure the atoms of each element balance out on both sides of the equation.
- Make sure all coefficients are in the simplest specific ratio. If needed, reduce the coefficients.
50 Best Examples Balanced Chemical Equations with Answers
- 1. Combustion of Methane (CH₄)
Unbalanced Equation:
CH4+O2→CO2+H2O
Balanced Equation:
CH4+2O2→CO2+2H2O
Answer: The equation should be balanced with 1 of carbon (C), 4 hydrogens(H) and 4 oxygens(O).
- 2. Synthesis of Water (H₂O)
Unbalanced Equation:
H2+O2→H2O
Balanced Equation:
2H2+O2→2H2O2
Answer: Both sides have 4 hydrogens and 2 oxygens.
- 3. Decomposition of Water
Unbalanced Equation:
H2O→H2+O2
Balanced Equation:
2H2O→2H2+O2
Answer: Equation balances with 4 H and 2 O on both sides.
- 4. Combustion of Ethane (C₂H₆)
Unbalanced Equation:
C2H6+O2→CO2+H2O
Balanced Equation:
2C2H6+7O2→4CO2+6H2O
Answer: Balances with 4 carbons, 12 hydrogens, and 14 oxygens on each side.
- 5. Combustion of Propane (C₃H₈)
Unbalanced Equation:
C3H8+O2→CO2+H2O
Balanced Equation:
C3H8+5O2→3CO2+4H2O
Answer: Balances with 3 carbons, 8 hydrogens, and 10 oxygens on each side.
- 6. Combustion of Butane (C₄H₁₀)
Unbalanced Equation:
C4H10+O2→CO2+H2O
Balanced Equation:
2C4H10+13O2→8CO2+10H2O
Answer: Balances with 8 carbons, 20 hydrogens, and 26 oxygens on both sides.
- 7. Combustion of Ethanol (C₂H₅OH)
Unbalanced Equation:
C2H5OH+O2→CO2+H2O
Balanced Equation:
C2H5OH+3O2→2CO2+3H2O
Answer: Balances with 2 carbons, 6 hydrogens, and 7 oxygens on each side.
- 8. Formation of Ammonia (NH₃)
Unbalanced Equation:
N2+H2→NH3
Balanced Equation:
N2+3H2→2NH3
Answer: Balances with 2 nitrogens and 6 hydrogens on both sides.
- 9. Decomposition of Ammonia
Unbalanced Equation:
NH3→N2+H2
Balanced Equation:
2NH3→N2+3H2
Answer: Balances with 2 nitrogens and 6 hydrogens on each side.
- 10. Reaction of Sodium with Water
Unbalanced Equation:
Na+H2O→NaOH+H2
Balanced Equation:
2Na+2H2O→2NaOH+H2O→2NaOH+H2
Answer: Balances with 2 sodiums, 4 hydrogens, and 2 oxygens on each side.
- 11. Reaction of Magnesium with Oxygen
Unbalanced Equation:
Mg+O2→MgO
Balanced Equation:
2Mg+O2→2MgO2
Answer: Balances with 2 magnesiums and 2 oxygens on each side.
- 12. Reaction of Iron with Oxygen
Unbalanced Equation:
Fe+O2→Fe2O3
Balanced Equation:
4Fe+3O2→2Fe2O3
Answer: Balances with 4 irons and 6 oxygens on both sides.
- 13. Reaction of Aluminum with Oxygen
Unbalanced Equation:
Al+O2→Al2O3
Balanced Equation:
4Al+3O2→2Al2O3
Answer: Balances with 4 aluminums and 6 oxygens on both sides.
- 14. Reaction of Zinc with Hydrochloric Acid
Unbalanced Equation:
Zn+HCl→ZnCl2+H2
Balanced Equation:
Zn+2HCl→ZnCl2+H2
Answer: Balances with 1 zinc, 2 hydrogens, and 2 chlorines on both sides.
- 15. Reaction of Calcium with Water
Unbalanced Equation:
Ca+H2O→Ca(OH)2+H2
Balanced Equation:
Ca+2H2O→Ca(OH)2+H2
Answer: Balances with 1 calcium, 4 hydrogens, and 2 oxygens on both sides.
- 16. Reaction of Sodium with Chlorine
Unbalanced Equation:
Na+Cl2→NaCl
Balanced Equation:
2Na+Cl2→2NaCl
Answer: Balances with 2 sodiums and 2 chlorines on both sides.
- Neutralization of Hydrochloric Acid and Sodium Hydroxide
Unbalanced Equation:
HCl+NaOH→NaCl+H2O
Balanced Equation:
HCl+NaOH→NaCl+H2O
Answer: One hydrogen (H), one chlorine (Cl), one sodium (Na), and one oxygen (O) are balanced on both sides.
- Decomposition of Potassium Chlorate
Unbalanced Equation:
KClO3→KCl+O2
Balanced Equation:
2KClO3→2KCl+3O2
Answer: Two potassiums (K), two chlorines (Cl), and six oxygens (O) are balanced on both sides.
- Formation of Ammonia
Unbalanced Equation:
N2+H2→NH3
Balanced Equation:
N2+3H2→2NH3
Answer: Two nitrogens (N) and six hydrogens (H) are balanced on both sides.
- 9. Decomposition of Calcium Carbonate
Unbalanced Equation:
CaCO3→CaO+CO2
Balanced Equation:
CaCO3→CaO+CO2
Answer: One calcium (Ca), one carbon (C), and three oxygens (O) are balanced on both sides.
- 10. Reaction of Iron with Oxygen
Unbalanced Equation:
Fe+O2→Fe2O3
Balanced Equation:
4Fe+3O2→2Fe2O3
Answer: Four irons (Fe) and six oxygens (O) are balanced on both sides.
- 11. Combustion of Ethanol
Unbalanced Equation:
C2H5OH+O2→CO2+H2O
Balanced Equation:
C2H5OH+3O2→2CO2+3H2O
Answer: Two carbons (C), six hydrogens (H), and seven oxygens (O) are balanced on both sides.
- 12. Decomposition of Hydrogen Peroxide
Unbalanced Equation:
H2O2→H2O+O2
Balanced Equation:
2H2O2→2H2O+O2
Answer: Four hydrogens (H) and four oxygens (O) are balanced on both sides.
- 13. Formation of Calcium Hydroxide
Unbalanced Equation:
CaO+H2O→Ca(OH)2
Balanced Equation:
CaO+H2O→Ca(OH)2
Answer: One calcium (Ca), two oxygens (O), and two hydrogens (H) are balanced on both sides.
- 14. Reaction of Aluminum with Hydrochloric Acid
Unbalanced Equation:
Al+HCl→AlCl3+H2
Balanced Equation:
2Al+6HCl→2AlCl3+3H2
Answer: Two aluminums (Al), six hydrogens (H), and six chlorines (Cl) are balanced on both sides.
- 15. Synthesis of Sodium Chloride
Unbalanced Equation:
Na+Cl2→NaCl
Balanced Equation:
2Na+Cl2→2NaCl
Answer: Two sodiums (Na) and two chlorines (Cl) are balanced on both sides.
- 16. Decomposition of Silver Oxide
Unbalanced Equation:
Ag2O→Ag+O2
Balanced Equation:
2Ag2O→4Ag+O2
Answer: Four silvers (Ag) and two oxygens (O) are balanced on both sides.
- 17. Combustion of Octane
Unbalanced Equation:
C8H18+O2→CO2+H2O
Balanced Equation:
2C8H18+25O2→16CO2+18H2O
Answer: Sixteen carbons (C), thirty-six hydrogens (H), and fifty oxygens (O) are balanced on both sides.
- 18. Synthesis of Calcium Carbonate
Unbalanced Equation:
CaO+CO2→CaCO3
Balanced Equation:
CaO+CO2→CaCO3
Answer: One calcium (Ca), one carbon (C), and three oxygens (O) are balanced on both sides.
- 19. Reaction of Zinc with Hydrochloric Acid
Unbalanced Equation:
Zn+HCl→ZnCl2+H2
Balanced Equation:
Zn+HCl→ZnCl2+H2
Answer: One zinc (Zn), two hydrogens (H), and two chlorines (Cl) are balanced on both sides.
- 20. Decomposition of Ammonium Nitrate
Unbalanced Equation:
NH4NO3→N2O+H2O
Balanced Equation:
NH4NO3→N2O+H2O
Answer: Two nitrogens (N), four hydrogens (H), and three oxygens (O) are balanced on both sides.
- 21. Combustion of Butane
Unbalanced Equation:
C4H10+O2→CO2+H2O
Balanced Equation:
2C4H10+13O2→8CO2+10H2O
Answer: Eight carbons (C), twenty hydrogens (H), and twenty-six oxygens (O) are balanced on both sides.
- 22. Reaction of Copper with Silver Nitrate
Unbalanced Equation:
Cu+AgNO3→Cu(NO3)2+Ag
Balanced Equation:
Cu+2AgNO3→Cu(NO3)2+Ag
Answer: One copper (Cu), two silvers (Ag), two nitrogens (N), and six oxygens (O) are balanced on both sides.
- 23. Decomposition of Sodium Bicarbonate
Unbalanced Equation:
NaHCO3→Na2CO3+CO2+H2O
Balanced Equation:
2NaHCO3→Na2CO3+CO2+H2O
Answer: Two sodiums (Na), two carbons (C), six oxygens (O), and two hydrogens (H) are balanced on both sides.
- 24. Reaction of Magnesium with Hydrochloric Acid
Unbalanced Equation:
Mg+HCl→MgCl2+H2
Balanced Equation:
Mg+2HCl→MgCl2+H2
Answer: One magnesium (Mg), two hydrogens (H), and two chlorines (Cl) are balanced on both sides.
- 25. Combustion of Glucose
Unbalanced Equation:
C6H12O6+O2→CO2+H2O
Balanced Equation:
C6H12O6+6O2→6CO2+6H2O
Answer: Six carbons (C), twelve hydrogens (H), and eighteen oxygens (O) are balanced on both sides.
- 26. Formation of Sodium Hydroxide
Unbalanced Equation:
Na2O+H2O→NaOH
Balanced Equation:
Na2O+H2O→2NaOH
Answer: Two sodiums (Na), two oxygens (O), and two hydrogens (H) are balanced on both sides.
- 27. Reaction of Calcium with Water
Unbalanced Equation:
Ca+H2O→Ca(OH)2+H2
Balanced Equation:
Ca+2H2O→Ca(OH)2+H2
Answer: One calcium (Ca), four hydrogens (H), and two oxygens (O) are balanced on both sides.
- 28. Decomposition of Lead(IV) Oxide
Unbalanced Equation:
PbO2→PbO+O2
Balanced Equation:
2PbO2→2PbO+O2
Answer: Two leads (Pb) and four oxygens (O) are balanced on both sides.
- 29. Reaction of Iron with Sulfuric Acid
Unbalanced Equation:
Fe+H2SO4→FeSO4+H2
Balanced Equation:
Fe+H2SO4→FeSO4+H2
Answer: One iron (Fe), two hydrogens (H), one sulfur (S), and four oxygens (O) are balanced on both sides.
- 30. Combustion of Acetylene
Unbalanced Equation:
C2H2+O2→CO2+H2O
Balanced Equation:
2C2H2+5O2→4CO2+2H2O
Answer: Four carbons (C), four hydrogens (H), and ten oxygens (O) are balanced on both sides.
- 31. Reaction of Potassium with Water
Unbalanced Equation:
K+H2O→KOH+H2
Balanced Equation:
2K+2H2O→2KOH+H2
Answer: Two potassiums (K), four hydrogens (H), and two oxygens (O) are balanced on both sides.
- 32. Decomposition of Calcium Hydroxide
Unbalanced Equation:
Ca(OH)2→CaO+H2O
Balanced Equation:
Ca(OH)2→CaO+H2O
Answer: One calcium (Ca), two oxygens (O), and two hydrogens (H) are balanced on both sides.
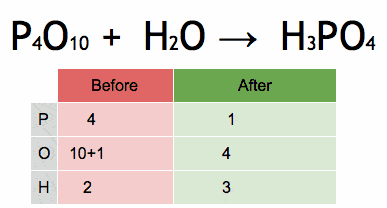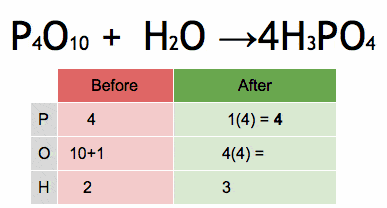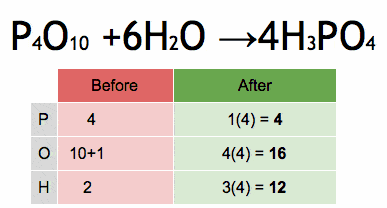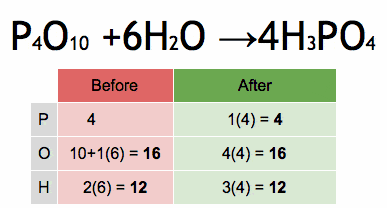
- 33. Formation of Carbonic Acid
Unbalanced Equation:
CO2+H2O→H2CO3
Balanced Equation:
CO2+H2O→H2CO3
Answer: One carbon (C), three oxygens (O), and two hydrogens (H) are balanced on both sides.
- 34. Reaction of Aluminum with Sulfuric Acid
Unbalanced Equation:
Al+H2SO4→Al2(SO4)3+H2
Balanced Equation:
2Al+3H2SO4→Al2(SO4)3+3H2
Answer: Two aluminums (Al), six hydrogens (H), three sulfurs (S), and twelve oxygens (O) are balanced on both sides.
- 35. Combustion of Benzene
Unbalanced Equation:
C6H6+O2→CO2+H2O
Balanced Equation:
2C6H6+15O2→12CO2+6H2O
Answer: Twelve carbons (C), six hydrogens (H), and thirty oxygens (O) are balanced on both sides.
- 36. Decomposition of Magnesium Carbonate
Unbalanced Equation:
MgCO3→MgO+CO2
Balanced Equation:
MgCO3→MgO+CO2
Answer: One magnesium (Mg), one carbon (C), and three oxygens (O) are balanced on both sides.
- 37. Formation of Ammonium Chloride
Unbalanced Equation:
NH3+HCl→NH4Cl
Balanced Equation:
NH3+HCl→NH4Cl
Answer: One nitrogen (N), four hydrogens (H), and one chlorine (Cl) are balanced on both sides.
- 38. Reaction of Lithium with Water
Unbalanced Equation:
Li+H2O→LiOH+H2
Balanced Equation:
2Li+2H2O→2LiOH+H2
Answer: Two lithiums (Li), four hydrogens (H), and two oxygens (O) are balanced on both sides.
- 39. Decomposition of Sodium Chlorate
Unbalanced Equation:
NaClO3→NaCl+O2\text{NaClO}_3 \rightarrow \text{NaCl} + \text{O}_2NaClO3→NaCl+O2
Balanced Equation:
2NaClO3→2NaCl+3O22\text{NaClO}_3 \rightarrow 2\text{NaCl} + 3\text{O}_22NaClO3→2NaCl+3O2
Answer: Two sodiums (Na), two chlorines (Cl), and six oxygens (O) are balanced on both sides.
- 40. Formation of Aluminum Oxide
Unbalanced Equation:
Al+O2→Al2O3
Balanced Equation:
4Al+3O2→2Al2O3
Answer: Four aluminums (Al) and six oxygens (O) are balanced on both sides.
- 41. Combustion of Pentane
Unbalanced Equation:
C5H12+O2→CO2+H2O
Balanced Equation:
C5H12+8O2→5CO2+6H2O
Answer: Five carbons (C), twelve hydrogens (H), and sixteen oxygens (O) are balanced on both sides.
- 42. Reaction of Potassium Permanganate with Hydrogen Peroxide
Unbalanced Equation:
KMnO4+H2O2→MnO2+O2+KOH
Balanced Equation:
2KMnO4+3H2O2→2MnO2+3O2+2KOH
Answer: Two potassiums (K), two manganeses (Mn), eight oxygens (O), four hydrogens (H) are balanced on both sides.
- 43. Decomposition of Calcium Sulfate
Unbalanced Equation:
CaSO4→CaO+SO3
Balanced Equation:
CaSO4→CaO+SO3
Answer: One calcium (Ca), one sulfur (S), and four oxygens (O) are balanced on both sides.
- 44. Reaction of Copper with Sulfur
Unbalanced Equation:
Cu+S→CuS
Balanced Equation:
Cu+S→CuS
Answer: One copper (Cu) and one sulfur (S) are balanced on both sides.
- 45. Combustion of Methanol
Unbalanced Equation:
CH3OH+O2→CO2+H2O
Balanced Equation:
2CH3OH+3O2→2CO2+4H2O
Answer: Two carbons (C), eight hydrogens (H), and eight oxygens (O) are balanced on both sides.
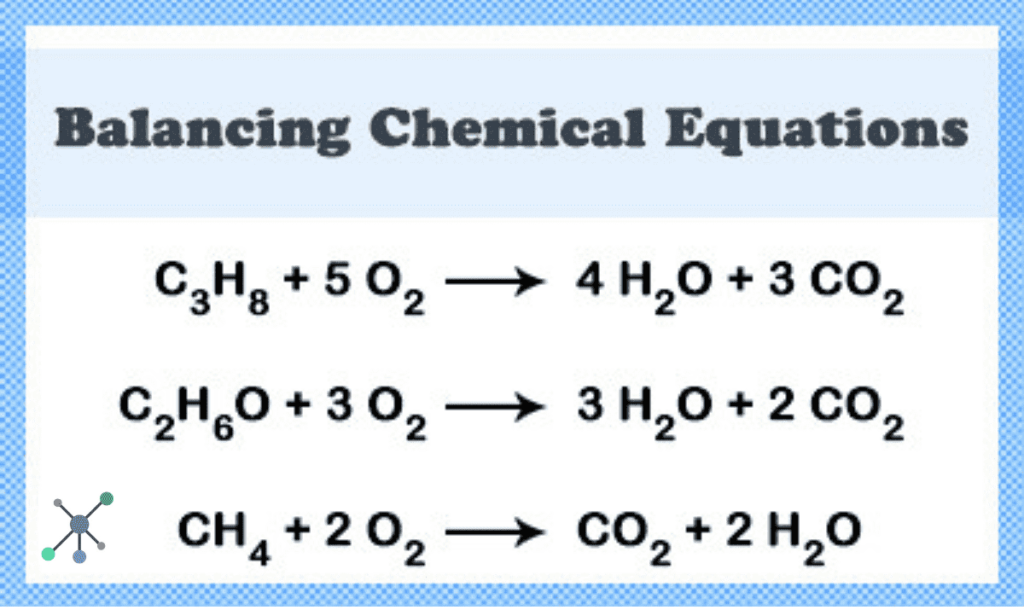
- 46. Reaction of Silver Nitrate with Hydrochloric Acid
Unbalanced Equation:
AgNO3+HCl→AgCl+HNO3
Balanced Equation:
AgNO3+HCl→AgCl+HNO3
Answer: One silver (Ag), one nitrogen (N), three oxygens (O), one chlorine (Cl), and one hydrogen (H) are balanced on both sides.
- 47. Decomposition of Aluminum Oxide
Unbalanced Equation:
Al2O3→Al+O2
Balanced Equation:
2Al2O3→4Al+3O2
Answer: Four aluminums (Al) and six oxygens (O) are balanced on both sides.
- 48. Formation of Phosphoric Acid
Unbalanced Equation:
P2O5+H2O→H3PO4
Balanced Equation:
P2O5+3H2O→2H3PO4
Answer: Two phosphoruses (P), eight oxygens (O), and six hydrogens (H) are balanced on both sides.
- 49. Reaction of Barium Chloride with Sulfuric Acid
Unbalanced Equation:
BaCl2+H2SO4→BaSO4+HCl
Balanced Equation:
BaCl2+H2SO4→BaSO4+2HCl
Answer: One barium (Ba), two chlorines (Cl), two hydrogens (H), one sulfur (S), and four oxygens (O) are balanced on both sides.
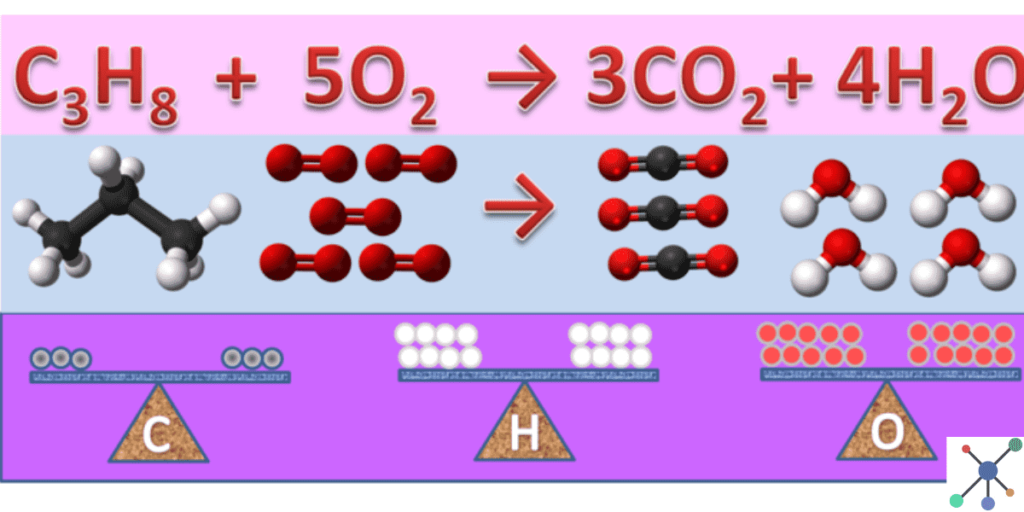
- 50. Combustion of Propane
Unbalanced Equation:
C3H8+O2→CO2+H2O
Balanced Equation:
C3H8+5O2→3CO2+4H2O
Answer: Three carbons (C), eight hydrogens (H), and ten oxygens (O) are balanced on both sides.
These are the list of 50 Examples Of Balanced Chemical Equations With Answers.
Also Read:
Positive Deviation From RAOULT’S LAW – Explaination
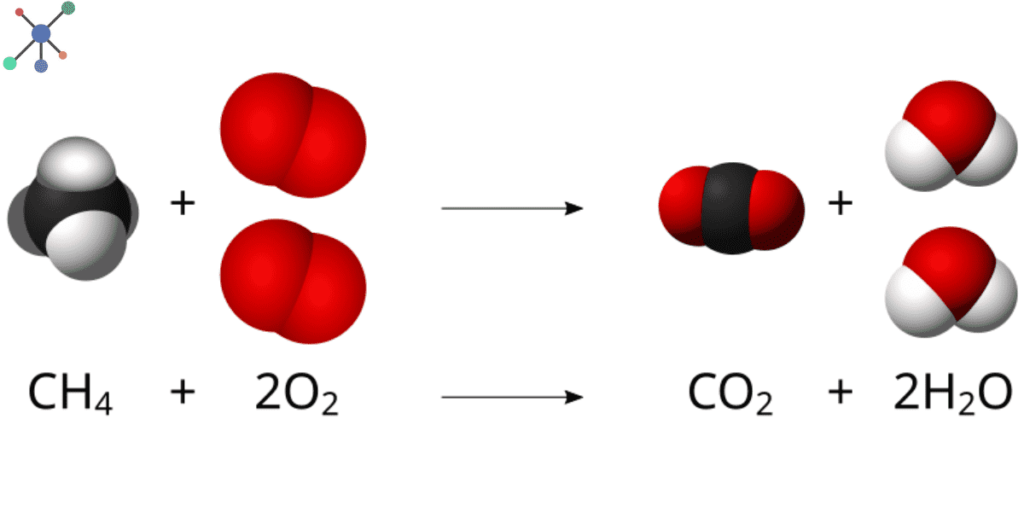
The process of balancing chemical equations must look formidable when one is introduced to it for the first time; though, as one gets to practice it, it becomes very easy.
So now, after having walked through these 50 examples you have not only mastered the balancing equations but also got a far better picture of what types of chemical reactions occur in your environment.
For those students who are in a process of preparing for the exam, or for those students who go in for revision of the lessons, these worked examples will be a great help to learn this quite important type of task.
Well, what you have to always bear in mind about chemistry is practice and nothing but practice.
Do more of the equations given above and you will realize that balancing equations is one of the easiest things you will ever do and very rewarding at that…
If the article on “50 Examples Of Balanced Chemical Equations With Answers” helped you a bit, kindly share with your family and friends.
Examples Of Chemical Equations With Answers for Combustion Reactions
When methane burns in the presence of oxygen (O₂), which results in the production of water (H2O) molecule and carbon dioxide (CO₂) molecule. This represents the breakdown of a compound.
Thus, one molecule of carbon dioxide and two molecules of water are created when two molecules of oxygen and one molecule of methane reacts with each other.
CH4+2O2→CO2+2H2O
Examples Of Chemical Equations With Answers for Decomposition Reactions
On heating hydrogen peroxide, it decomposes into two molecules, oxygen gas (O2) and water (H2O). This reaction depicts the breakdown of one molecule into two more basic ones.
Thus, two molecules of hydrogen peroxide decompose into one oxygen molecule and two molecules of water.
2H2O2→2H2O+O2
Examples Of Chemical Equations With Answers in Redox Reactions
When zinc is added to the copper sulfate solution, a reaction occurs where the zinc is oxidized, that means it loses electrons and the copper reduces, thus it gains electrons.
In this reaction, zinc replaces copper, creating zinc sulfate and solid copper.
Zinc loses electrons, that is oxidation reaction. While copper gains them, that is, reduction reduction reaction, making this a classic redox reduction reaction.
Zn+CuSO4→ZnSO4+Cu
Examples Of Chemical Equations With Answers for Organic Reactions
When ethene reacts with hydrogen in the presence of a catalyst such as nickel, a hydrogenation reaction occurs. Transforming ethane into ethane (C2H6).
This is a classic organic reaction where an unsaturated compound becomes saturated.
Thus, an ethene molecule joins with a hydrogen molecule to form ethane, a simpler and more stable form of alkane.
C2H4+H2→C2H6
How to Balance a Chemical Equation With Example?
Gee! Have you ever puzzled over a chemical equation and been totally lost? I am! In my chemistry class, balancing the chemical equations was my worst enemy.
One thing is clear. The task is not so challenging. Truly, it gets you to a point where it’s almost like solving a puzzle, you see the gradual process and then you can do it.
I can still recall the time when I just “got” the whole concept. I was really struggling with a specific problem when my teacher shared with me a very simple, step-by-step approach that changed everything.
What seemed impossible to me before now became pretty achievable and even fun. Right now, I’m super excited to kind of give you the same insights.
If you are a student thinking about chemistry homework problems or just curious about the science behind chemical reactions. I will answer your questions.
In this article, I am going to guide you through the process of balancing chemical equations.
The first thing to be addressed is the most basic form of balancing; we are going to then, break down the components, and then we are going to move in a process, which is both simplest and virtually foolproof also.
Of course, I will be mentioning some methods and pitfalls of the advanced level during the course of the lesson so that by the end, you really do have enough learning, as well as not to be afraid faced by any chemical equation. Are you prepared to transform your chemist capabilities? Let’s start!
Understanding the Chemical Equation
Chemical equations are central guidelines in science, assertions surveys a high school level chemistry teacher and a teacher of educational chemistry.
In this article we will learn 50 examples of balanced chemical equations with answers and will know how to balance them effectively.
In this division, I will elucidate the elements of chemical equations and then bring in some arguments as to their significance in the field of chemistry.
Definition of a Chemical Equation
A chemical equation is a symbolic picture of a chemical reaction. It shows the reactants (the starting substances) and the products (the resulting substances) using chemical formulas.
This is the piece that I often use as a reminder to my students. Get them to imagine it as a recipe of a chemical reaction.
2H₂ + O₂ → 2H₂O
In this equation, the reactants are hydrogen (H₂) and oxygen (O₂). Besides the product, water (H₂O) is also the result. The arrow (→) denotes the direction of the reaction.
While educating, and I can make clear that through the comparison of a chemical equation to a mathematical equation (usually given), I am able to emphasize my points:
| Chemical Equation | Mathematical Equation |
| Chemical symbols and formulas are used by it. | Numbers and mathematical symbols are used by it. |
| It Represents a chemical process. | It Represents a numerical relationship. |
| The Coefficients show quantity ratios. | The coefficients must multiply each other. |
| The Arrow (→) means it is a reaction direction. | The Equals sign (=) shows that two terms are equal. |
Explanation of Law of Conservation of Mass
This lesson centers on the significance of the law of conservation of mass when balancing chemical equations.
One of the very first teaching points I stress to my learners is the importance of understanding the basics of chemical equations; this law serves as a sturdy support base on this subject.
The law of conservation of mass essentially asserts:
- Matter is neither brought into existence nor eradicated during a chemical transformation.
- Consequently, the total mass amount found in any reaction remains conserved regardless of the process the substances undergo.
Methane Combustion as an Example
CH₄ + 2O₂ → CO₂ + 2H₂O
In the above equation where we see a reaction, it’s easy to marvel at how the law holds sound until proved to the letter. Let’s develop the law of conservation of mass in the balance equation with the following development:
- In the (CH₄) – reactants on the left-hand side, there are 1 neutron, 4 protons, and 4 electrons of hydrogen and only 1 neutron, 4 protons, and 4 electrons of methane’s carbon.
- The other side of the equation, the products consist of 1 neutron, 4 protons, and 4 electrons of hydrogen and 1 neutron, 4 protons, and 4 electrons of oxygen on the right-hand side.
The mass is conserved, and no atoms are created or destroyed. They are just re-arranged.
In a nutshell, I usually come up with this analogy. Let’s say that you have a bunch of Lego blocks.
You can create various shapes, but the number of blocks is constant. On a chemical reaction, atoms are merely rearranged but the total number remains the same.
Importance of balanced chemical equations in chemistry with examples
Having thus far learned the basics, now we are ready to understand and grasp the importance of the balanced equations.
I can say, from my time in the search field and the classroom, that balanced reactions are the most utilized tools in the studies of chemistry and beyond.
Precise predictions:
The productive balanced equation gives us the exact amounts of reactants we need plus the founded products.
This technique is quite essential for the industries that optimization and cost-cutting are the main concerns. Especially, it is for the industries that apply this method.
Stoichiometric calculations:
The chemical equations based on the balanced ones are used to calculate quantitatively the substances that take part in a chemical reaction.
Knowing the reaction mechanisms:
A balanced equation is used to convey information on the molecular level on the way the reactions occur that are the basis for the development of new chemical methods and the understanding of complex biological reactions.
Environmental impact assessment:
In environmental chemistry, balanced equations are an excellent tool to comprehend the impact of chemical reactions on our environment. They give us the capacity to measure the changes in biomass during a process.
Safety in chemical handling:
The correct ratios of reactants and products should be known for the safe handling of materials, irrespective of the place, be it a factory or a warehouse.
Let me give you a simple illustration that helps explain this point further:
This is the example of balanced chemical equation for the production of ammonia using the Haber process:
N₂ + 3H₂ ⇌ 2NH₃
From this balanced chemical equation, it can be deduced that:
1. For one mole of nitrogen, we need exactly three moles of hydrogen.
2. The reaction will produce two moles of ammonia per mole of nitrogen.
3. The presence of a double arrow (⇌) indicates that it is reversible.
Industrial chemists would have been clueless without this balanced equation about:
1. How much reactant to charge into the reactor
2. The amount obtained
3. How best to maximize efficiency in the process
From my experience, most students usually find it difficult balancing equations when they start learning.
However, once they get its importance and see how it applies in real life scenarios; their motivation towards understanding it improves significantly.
One way I have found particularly effective at reinforcing this concept is through conducting hands on experiments such as simple reaction between baking soda and vinegar.
For an instance, I often have my students perform a simple reaction between baking soda and vinegar:
NaHCO₃ + CH₃COOH → CH₃COONa + H₂O + CO₂
They would physically prove the law of conservation of mass by measuring the masses of reactants and products as well as see how necessary a balanced equation is for predicting reaction outcomes.
We will examine more closely the constituents of chemical equations and how to balance them step wise.
These basics are very important, because they enable students to delve into higher level chemistry concepts and apply them in real life situations.
Importantly, a well-balanced chemical equation does for a chemical reaction what a good sentence does for writing – tells it coherently and completely.
We should remember that every time you encounter or create an equation in which there is equal number of atoms on both sides, you are actually unlocking some secrets about the matter around us.
Step-by-Step: How to Balance a Chemical Equation with Answers (Examples Included)
After looking at the chemical equation components, we will discuss how to balance these equations in this step by step process. As one who has spent years dealing with chemical equations,
I tell you that being proficient in this art is a key requirement for anyone who wants to be a chemist or just a science fan.
Write the unbalanced chemical equation
Initially, before you can proceed to balance a chemical equation, you must be able to write the unbalanced equation.
This is where I begin with the reactants at the left hand side of an arrow and products on the right hand side. It is important to make sure that all the chemical formulas are right before going further.
To demonstrate this point, let us consider the reaction between hydrogen and oxygen to form water:
H2 + O2 → H2O
This equation is off-balance in terms of equality, but it provides a perfect platform upon which the next steps are built.
Count atoms on both sides
The first thing I do is write the unbalanced equation = when it comes out of the bottle basically.
Then after, I count how many of each type of atom there are on each side of the equation, because then you can see what elements need to be balanced most easily.
Let’s count the atoms in our example:
| ELEMENTS | REACTANTS | PRODUCTS |
| Hydrogen | 2 | 2 |
| Oxygen | 2 | 1 |
This is apparent, hydrogen atoms are equal (2 on each side), but oxygen atoms are not (2 on the left, 1 on the right).
Add coefficients to balance atoms in an chemical equation
Next I look at which atoms are not balanced and then I put coefficients in front of the formulas to balance these not balanced atoms, always starting with the most complicated and/or the molecule with the most atoms.
In our case, we need to balance oxygen atoms. My first step will be to put 2 in front of H2O:
H2 + O2 → 2H2O
Now let’s recount our atoms:
| ELEMENTS | REACTANTS | PRODUCTS |
| Hydrogen | 2 | 4 |
| Oxygen | 2 | 2 |
Good! The oxygen atoms are balanced. But we made a mess with the hydrogen atoms.
To do that, I will multiply H2 with 2.
2H2 + O2 → 2H2O
Let’s do our final count:
| ELEMENTS | REACTANTS | PRODUCTS |
| Hydrogen | 4 | 4 |
| Oxygen | 2 | 2 |
Perfect! All the atoms are balanced now in both the sides of equation.
Check and adjust the Chemical Equation to further balance it
My final step is the check and adjust if necessary. I always double check to see that: –
- For each element the number of atoms is the same on both sides of the equation.
- The coefficients used are the smallest whole numbers possible.
- There are no fractional coefficients.
In our example, 2H2 + O2 → 2H2O, all the criteria are met so we can be confident it is balanced.
Now let’s do a more complex one to make sure you’ve got it. We’ll balance the reaction for the combustion of propane:
C3H8 + O2 → CO2 + H2O
Steps, we need to follow:
Count the atoms in the equation:
| ELEMENTS | REACTANTS | PRODUCTS |
| Carbon | 3 | 1 |
| Hydrogen | 8 | 2 |
| oxygen | 2 | 3 |
Add coefficients to balance:
To start, we shift carbon and multiply CO2 by 3:
C3H8 + O2 → 3CO2 + H2O
Now we will balance hydrogen by changing the coefficient of H2O = 4.
C3H8 + O2 → 3CO2 + 4H2O
Lastly-how to balance of our oxygen. On the right side there are ten oxygen atoms (six in CO2, and four in H20); this means that we need five O2 molecuels on the left:
C3H8 + 5O2 → 3CO2 + 4H2O
Check and adjust the Chemical Equation to make sure it is Balanced.
Let’s do our final count:
| ELEMENTS | REACTANTS | PRODUCTS |
| Carbon | 3 | 3 |
| Hydrogen | 8 | 8 |
| oxygen | 10 | 10 |
So, we have balanced the chemical equation finally!
Let me show you the significance of Balanced Chemical Equations, with a few examples to go along.
Significance: Balanced Chemical Equations
Balanced equations are inescapable whilst doing calculations on this scale, such as required for industrial chemistry (to work out how much of each reactant you need and the quantities of products produced).
As for an example, in the manufacturing of ammonia (Haber process)
N2 + 3H2 → 2NH3
So this balanced equation here tells me that it takes 3 molecules of hydrogen to make 2 molecules of ammonia for every molecule of nitrogen.
Such info is important in the optimization of commercial processes and to get a better yield.
For practical reasons a step-by-step process to this is generally shown even though some complex reactions get more into it.
Nevertheless, being able to do this simple method is a good starting point for working out harder equations.
From my practice, few tips will help the one that begin with balancing chemical equations.
For practice, I usually advise solving several examples: simple reactions at first and then the more complicated ones.
If you do this, it will improve your confidence as well and allows you to intuitively go through the step of balancing.
Here are some reactions you can attempt to balance in practice:
- Zn + HCl → ZnCl2 + H2
- Fe + O2 → Fe2O3
- CH4 + O2 → CO2 + H2O
- Al + H2SO4 → Al2(SO4)3 + H2
Remember, the main steps to balance a chemical equation are:
- Write the unbalanced equation
- Count atoms on both sides
- This is used to make coefficients by multiplying the appropriate number and then balancing atoms.
- Check and adjust
As you go through these examples, your speed and accuracy will increase. Don’t get disheartened if there are some equations that you can not work out the aspects of at first, it’s all part of how we learn.
In later sections, we dive into more advanced techniques to balance equations that are a bit tougher and you may see in your studies or work.
And this will be ground on where you can solve the most complicated chemistry reactions and process with ease.
Why Should Chemical Equations Be Balanced?
Balancing chemical equations can look to be an exhausting task, but it is actually quite essential. Balancing an equation is fundamentally about making sure that everything adds up.
In a chemical reaction, atoms cannot be created or destroyed randomly.
The law of conservation of mass states that what goes in must come out, hence the number of atoms for each element must be equal on both sides of the equation.
If you consider it akin to a recipe. You need a specific quantity of flour, sugar, eggs, etc. for preparing cake.
Your cake wouldn’t turn out properly if an egg vanished or an extra cup of flour appeared out of nowhere. Similar to this, an imbalanced chemical equation obscures the real image of the reaction’s dynamics.
By balancing the equation, chemists can accurately predict the quantity of each substance required and the the amount of product that will be produced.
This is critical whether you’re in a lab mixing chemicals or a manufacturing plant scaling up a process.
It maintains everything in balance, reduces waste, and promotes safety. So, while it may seem like a little step, balancing equations is a critical aspect of getting chemistry right.
What is the Trick to Balance a Chemical Equation?
So, what’s the trick to balancing chemical equations? The good news is that it isn’t as complicated as it appears at first. Here’s an easy, step-by-step method that works every time.
Taking a chemical equation step by step is essential to its equilibrium. Start by posting each of the fixings in the response. On both sides, count the number of atoms in each element.
Modify the coefficients to begin balancing each element one at a time, starting with those that only occur in one molecule on each side.
Because they are commonly found in many compounds, oxygen and hydrogen should be left till last. Finally, examine your work to ensure it is balanced.
With experience, this procedure provides a simple and reliable way to balance any chemical equation.
Can any Chemical Equation Be Balanced?
Yes, any chemical equation that accurately defines a chemical process can be balanced.
The act of making sure each element has the same number of atoms on both sides of the equation, as needed by the mass conservation law, is known as “balancing”.
This law says that matter cannot be formed or destroyed, therefore every component from the reactants must appear in the objects.
However, always keep in consideration that complicated molecules or multistep processes can make it more difficult to balance some equations. Patience and caution are both necessary in these conditions.
If an equation appears hard for it to balance, it could indicate that the reaction was wrongly written or failed to take place under the circumstances that were specified.
However, so long as the reaction is accurate, the equation may always be balanced using suitable factors. It’s simply a matter of exercising and implementing a logical procedure.
We’ve gone through many different kinds of chemical reactions in this post on “50 Examples Of Balanced Chemical Equations With Answers“, showing you how to balance them in step-by-step method.
Chemistry will seem a little more manageable if you can better balance equations by comprehending these examples.
Balancing chemical reactions will become second nature if you practise with these 50 examples of balanced chemical equations with solutions.
Share this article with your beloved family and friends.
