In This Article We Are Going to Discuss About Variation of Conductivity and Molar Conductivity with Concentration.
I mean, well… think about how electricity can be affected by adding more or less of a given substance? All right, let us get started with the ‘Variation of Conductivity and Molar Conductivity with Concentration’.
This subject is about the conductivities of common table salt (sodium chloride), sugar, and other substances—what makes them good or poor conductors of electricity.
Now let us consider how this Decrease is Affected With Concentration Variation (Enabled Type) and come with some inferencesfootnote- need to know so many things?
Excited to do your own exploration of the thrilling world of conductivity? After that, practice has been the main thing to do… but how important is concentration here?
Let’s know.
Variation of Conductivity and Molar Conductivity with Concentration: Introduction
They’ll call it “conductivity” when we talk about how electricity moves through things. Conductivity tells us how easily something can conduct an electric charge.
The materials that are really good at this would be metals, while some other in the case of rubber.
However, if we take liquid conduction in account and the way a different factor is involved when considering how well one conducts electricity or not… that thing called “concentration” which means: how much substance you add into it.
Chemists frequently work with solutions, a mixture in which one substance (salt or acid) dissolves into another (water). Conductivity increases with the concentration of this substance in water.
But the interesting thing goes last, as you keep increasing your concentration; there is a difference way that conductance changes. It’s on the up, and sometimes down!
Another term, molar conductivity. It teaches us just how conductive every particle (or molecule) in the solution is. Molar conductivity, Like Conductivity molar conductivity also changes with concentration.
This is important in chemistry, because it allows us to predict how different substances will behave when dissolved (which can be interesting for many applications — from batteries to cleaning products!)
The Conductivity of Strong Electrolyte
Ever wonder why some liquids can carry an electric current while others cannot? The inside is all that matters!
Strong Electrolytes — A Different Angle Salt is an example of a strong electrolyte because when it dissolves in water, salt breaks apart into tiny particles known as ions.
Those ions are kind of like electrons but aqueous — they can float around in the water with an electric charge. This is how water dissolved with electrolyte can conduct electricity.
As for an example, when a grain of table salt dissolves in water it separates into sodium (Na⁺) and chloride ion itself.
These ions enable electricity to flow in the solution, akin how wires carry current from one part of your house to another.
Strong electrolytes differ from weak ones because they completely dissociate in water, making the solution much more conductive than just having ions.
Introduction In this first chapter we will examine the concept of just what is a strong electrolyte, why it behaves as such an excellent conductor (and insulator), and how inter-ionic interactions differentiate them from other substances. Lead into the fascinating Ion and electricity world
Molar Conductance
Molar conductance can seem like a big, complex word but in reality it is pretty easy to understand when we break it down. Picture this as a solution, such as saltwater (which is water with the solute of table salt dissolved in the solvent). These ions is then light enough that they are free to move and carry electric current.
So, molar conductance tells us about the movement of these ions in solution. Note something here, it is akin to counting of free movement for cars across a road. If the road is wide and empty, cars will drive freely through it. They have problems because if the road is narrow and crowded.
Molar Conductance: Molar conductanace tells us about some of the movement of ions in solution which help to conduction electricity easily.
It is based on two factors i.e., the number of ions in the solution and conductivity capacity of the solution. The higher concentration of ions and better movement the compound has, molar conductance is also high.
This concept can be used by scientists to gain more information about various solution and their conductivity(extension cord). Chemists use it to help determine the strength of an acid or a base, and how things will behave when you fire them into water.
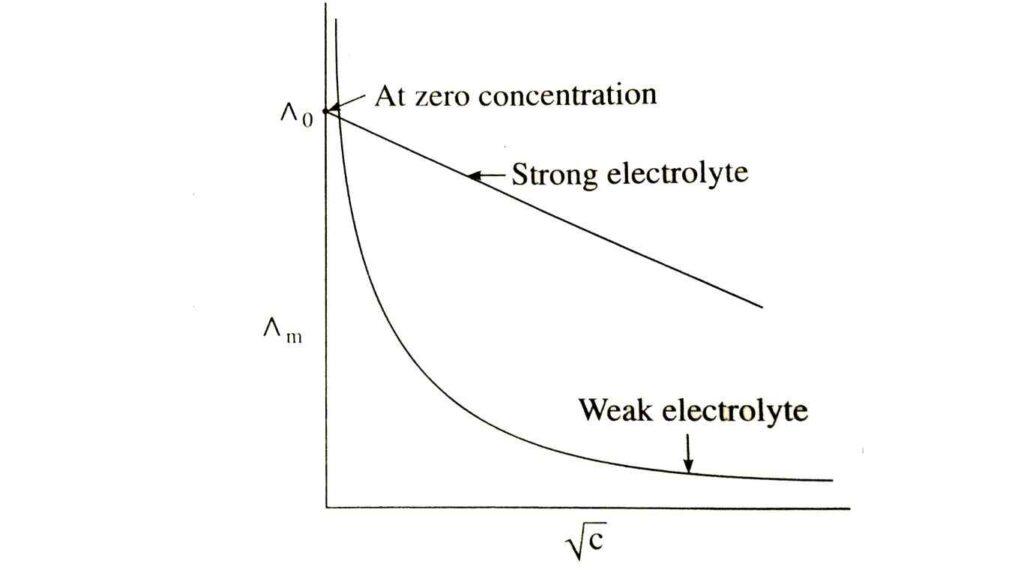
Molar Conductivity VS Concentration Graph
The molar conductivity is a measure of the ability for solution to conduct electricity using ions. Ions: When things like salt is dissolved in water, they turn into small charged molecules called ions. These ions are free to flow around and this promotes electricity.
So, when we discuss the “Molar Conductivity vs. Concentration Graph” is just showing us that how these ions now looses their capability to conduct electricity as continuously changing amount of substance added in water
Generally, molar conductivity does not remain constant as the nature of the solution changes from more dilute to less concentrated ions are at higher concentrations because ions occupy space, any when they do there is less water for each individual ion.
But at the molar conductivity increases because of when it is more dilute, there ions have space to move around.
It is also useful for scientists as it gives an idea about how the ions behave at various concentrations. We can learn something about the nature of the substance in solution and how it passes electricity under a variety of conditions by studying that graph.
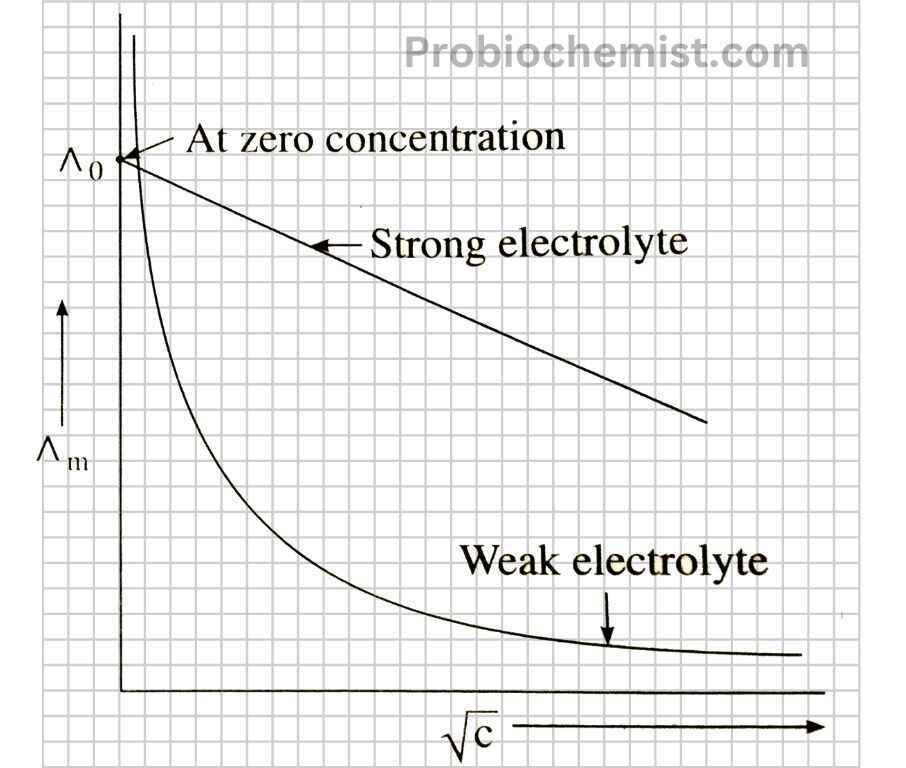
What is Conductivity in Chemistry
In chemistry, an identical way of being is how conductive a material can be. If you will, a highway for electricity to pass through.
Materials like metals are excellent conductors of electricity, kind off how smooth roads allows cars to speed their way downstream.
These materials are known as “conductors”. A third example would be those types that perform very badly, just like bumpy or blocked roads are preventing cars from moving — such as rubber and plastic.
Saltwater (and liquids generally) allows electricity to flow through it thanks in part of the ions, which are small charged particles. If a material allows electricity to flow with little resistance, we say that material has high conductivity. If it does not, the conductivity is low.
Molar Conductance
Molar conductance is the specific for of measuring conductivity of an electrical in a liquid state, coming from dissolved ions and depending on how well it can carry currents.
Suppose you have salt in water. A charge is separated over the salt and it then breaks up into small pieces called ions, which are mobile. These ions facilitate the electric charge to flow through water. This helps us tell how easily these ions can move (Molar conductance).
This will vary depending on the salt in water (of how big are ions). What helps to carry the electric current is good water movement. The fishing line makes scientists able to follow the moving of all kinds of different chemicals in water, things like acids and salts and bases.
Also Read:
Poisson’s Ratio in Thermodynamics – Derivation
Biochemistry Analyzer Uses and Principle: A Comprehensive Guide
Haloalkanes and Haloarenes Handwritten Notes | Class 12 | Pdf
Structure of Atom Class 11 PPT
Factors Affecting the Variation of Conductivity and Molar Conductivity with Concentration
However, can we do this for the variation of conductivity and molar conductivity with concentration which is how well it conducts electricity in a solution?
Conductivity is the measure of how much electric current can be passed through a solution and molar conductivity defines that one mole entity which carries with it all this electricity.
This is what happens with concentration —
In general, conductivity increases with concentration. This is because so many more ions (tiny charged particles) are there to conduct the electricity. But it does not increase indefinitely — at sufficiently high concentrations the ions start to run into each other a little bit and obstruct things.
However molar conductivity decreases at higher concentration. Why? Now the more crowded space, especially when ions get “neutral”, means it is harder for each one to do its job of carrying electricity.
When the number of ions present is very limited (super dilute solutions), molar conductivity will be higher, because ions experience no opposition in movement. This study gives scientists insight into how substances interact in solutions and application of them on a global scale, such as batteries or cleaning supplies!
How Does Concentration Affect Conductivity?
If you add something to water that already happens, like salt or sugar it splits into ions (those little charged pieces we mentioned earlier). Similar to that in which these ions are hand across a very sophisticated dance floor but also, help carry the electrical current.
Low Concentrations:The low no of ions are present,so the conductivity is very less. Just imagine that there are only some cars traffic on a road, which means the intermediate device is not busy.
When At High Concentrations: The more salt (or some other dissolved substance in the water) you add, the greater number of ions As the number of ions increase, more current can flow which drives up the conductivity. Then you get the electrical portion, it is like adding more cars on a road—more traffic!
How Does Concentration Affect Molar Conductivity?
This is somewhat distinct from typical conductivity. It shows us how conductive each ion is to the free flow of electricity.
Low Concentrations: When ions are present at low concentrations, all the ions can move freely.
Note: In other words, they conduct electricity in those species, and the molar conductivity is high. It is the equivalent of a completely open highway that allows fast and unimpeded traffic.
So, High Concentrations: Higher concentrations are where things start to get a little tricky. It bumps with another ports because it has more ions in the water.
It means it is more difficult for charges to move around and electricity cannot flow as easily.
Therefore, while in the first case overall conductivity increase results it leads to a decrease in molar conductivity. Traffic jams, in the same way that too many cars on a road create congestion.
Variation of Conductivity and Molar Conductivity with Concentration, in simple words, is a measure of how good electricity can pass through any liquid and molar conductivity tells us about total dissociation even individual ion helps.
As a liquid becomes more and more of an item is added, what happens to its concentration? The greater the concentration, more ions are present, thereby increasing conductivity.
However, as the concentration increases further and ions come close by enough to interfere with each other, then molar conductivity decreases.
These concepts help scientists to apply their solutions into real-life applications like batteries and water purification systems!
Factors Affecting Electrolyte Conductivity
Conductivity of an electrolyte itself indicates how good the solution is in conducting electricity. So, what is it that can give some solutions an advantage over others at this?
There are a few things that affect how effective the electrolyte actually is at conducting electricity, including the Variation of Conductivity and Molar Conductivity with Concentration.
Strong & Weak Electrolytes: Electrolytes are of two types. A good example of a strong electrolyte is table salt, sodium chloride (NaCl), which dissolves in water almost completely into its ions.
Because acetic acid is a weak electrolyte, it does not dissociate very much and therefore isn’t as good of an electric conductor.
Concentration: The concentration of electrolyte in the body fluid also affects greatly. A solution containing more ions (charged atoms) will conduct electricity better than a solution with fewer ions.
This is where the Variation of Conductivity and Molar Conductivity with Concentration comes into play. Saltwater, for instance, has more ions than pure water, and salt is a good conductor of electricity.
Temperature: Ions in the solution move more quickly with increasing heat. This makes the solution more conductive, and therefore it is efficient to carry out current. This is why a warm solution will often conduct electricity better than a cold one.
Type of Solvent: The solvent in the liquid where the electrolyte is dissolved affects its conductivity. A majority of electrolytes are more soluble in water, as it assists them in dissolving into ions easily. Liquids that are not good for dissolving electrolytes, like oil, do not conduct electricity.
Electrode Separation: These are the points where a solution connects to its circuit. The more distant they are, the harder it is for charge carriers to move from one side of the device (cathode) to another side (anode), hence ion transport becomes less.
How Temperature, Pressure, and Solvent Affect Conductivity and Molar Conductivity
Second is that it tells us how well substances – salts, for instance—can conduct electricity in solution—that they purify—usually water. Now let us discuss how the temperature, pressure, and solvent can affect the Variation of Conductivity and Molar Conductivity with Concentration!
Heat: A solution’s particles move more quickly when it is heated. This facilitates the flow of electric current by making it simpler for charged particles, or ions, to collide.
Therefore, the conductivity of the solution often increases as the temperature rises. So what happens when the temperature increases is that it usually also rises, conductivity of your solution.
Pressure, for most things, pressure doesn’t change a solution much with respect to conduction. But when we’re talking about gases in a liquid, then extra pressure could get some more gas into the liquid and that would likely have at least a little effect on conductivity. But typically not as important as temperature.
Solvent: the liquid you dissolve something in. Water is the most common one. If you dissolve it in a different solvent, as with alcohol, the conductivity of that material may well change. This is because not all solvents are good at conducting ions.
So in other words, temperature makes stuff faster, pressure can be a dial but not that much, and the solvent decides how easy these ions are allowed to move! Understanding the Variation of Conductivity and Molar Conductivity with Concentration helps us see how these factors play their part in conductivity.
Practical Applications of Conductivity and Molar Conductivity Variations in Industry
Have you also wondered why your phone charges swiftly or a little bit fine; the taste of water changes variously? Much of this comes down to something called conductivity.
It measures the conduction ability of a substance. Think of it as a highway for electric charges to travel at high speed from one location to another.
In other words, when discussing the Variation of Conductivity and Molar Conductivity with Concentration, we are looking at how much the ions (charged particles) in a solution will carry electrical charge, especially under dilute conditions.
Conductivity and molar conductivity are major uses in industries. Join me as we enter the world of packages to understand how they are used and why it matters!
Water Treatment
One of the major functions among many is measuring conductivity in water treatment plants. Do you know, we have been listening since childhood that our water should be clean and pure?
Conductivity ensures this. Water with an abundant concentration of dissolved salts (ions) is a better fan of electricity than pure water, which comes in handy because sometimes you do not want it to conduct electricity.
Water pollution or impurities are signified by high conductivity in water. Conductivity measurements are used in the monitoring and control of ion levels for water treatment, such as the preparation of drinking water.
They can check their water at any time, and when the conductivity is too high, they know it’s time to open that tap so some unwanted ions are left behind, leaving clean purified water.

Battery Manufacturing
Heard from these few girls after all (Part 3). Ever wonder why some batteries last longer than others?
The reason batteries can hold a charge and then release energy is due to how freely the ions inside are able to move around. Here comes into play, the Variation of Conductivity and Molar Conductivity with Concentration.
The molar conductivity, especially in the production of lithium-ion batteries, is an important issue during battery manufacturing. In fact, the smooth movement of these ions is a delicate balance that engineers and scientists must strike with just enough electrolyte (liquid solutions containing molecules), but not too much.
This is because the ions wouldn’t be able to move as easily, which would either severely limit battery life or compromise performance.
Pharmaceuticals
Drugs should be pure, safe, and of quality. Conductivity measurements are used to verify the proper amount of ingredients in pharmaceutical production.
If the conductivity is too great, a solution might not be mixed correctly or have impurities in it. This way, by monitoring conductivity rigorously, companies can verify that the medication you’re having is of the best quality.
Food and Beverage Industry
Do you realize that even the food and drinks tested and controlled for being safe to consume are first chemically divided, mixed with different things (look at what happens from stalks of grains) so they would be more durable?
The Variation of Conductivity and Molar Conductivity with Concentration also has a key factor here. Conductivity is measured to check the quality of milk in one of its applications (for the dairy industry).
High conductivity would indicate that the milk may have become adulterated with salt or other things.
Conductivity is used in the control of the salt, sugar, or other ingredients required for the appropriate taste and drink safety.
Corrosion Monitoring
In industries such as oil, gas, and construction, there is a big challenge of metal corrosion, specifically metal rusting, which will, in turn, break down the structure or equipment.
Conductivity of solutions around the metal is a method used to observe corrosion. Higher conductivity could indicate that ions may be interacting with the metal, resulting in rust.
When the conductivity of the water is controlled, still in cathodic protection, we can measure this so that companies can anticipate steps to stop corrosion. Save time and money!
Therefore, every time you charge your mobile phone or drink some beverages (e.g., a soda) and drink water, just remember we have properties of conductivity all the way at IIT Jodhpur securities park!
From maintaining safe clean water to ensuring your phone battery is optimal, conductivity and molar conductivity make the things that we depend on work smoothly.
Applications of Conductivity in Real-World Systems
Now, conductivity sounds like a jumbo word but actually this is something that we see and use every day! Conductivity is how easily things let electricity (electricity conductivity) or heat (thermal conductivity) pass through them.
Now, allow me to explain how the property of conductivity benefits us in real-world applications.
- Technology: An alternative to electronics, consider your preferred technological gadgets like tablets or phones, even video game machines. Contained within these devices are small circuits that must function correctly to ensure everything goes as planned. Your devices need electricity to light up and do awesome tricks, but they cannot conduct any without conductivity!
- Cooking — have you ever tried stirring a pot that’s on the stove with a metal spoon? This is because metals are excellent conductors of heat. These fan work to distribute the heat evenly so that your recipes get consistency and are cooked properly. Bad conductivity = half-cooked food again!
- Conductivity and water quality: Did you know that the physical properties of material can tell us whether water is clean? Scientists use the Variation of Conductivity and Molar Conductivity with Concentration to determine whether water is contaminated by measuring how much of chemical substances dissolve in it. Conductivity is a measure of how well water carries an electrical charge, and we all love clean drinking water, right!
- Power Lines: The electricity that runs to your home is sent through power lines. These are constructed with highly conductive material to allow the electricity to travel long distances without losing much power.
Conductivity is almost a superpower of most things in the world. It’s what makes our gadgets operate, cooking convenient, water sanitary, and carries power all the way to your living room. Isn’t that amazing?
How Conductivity Measurements Are Used in Industries Like Water Treatment, Pharmaceuticals, and Food Production.
A conductivity measurement is a kind of special tool that tell to people in different occupations, whether they know this word or not, so everything works out perfectly.
Please imagine a glass of water in front of you. If you stick in a special probe, there is some level of which the water can conduct electricity. This is known as conductivity and it tells us a great deal about what may be in the water.
It is very important in water treatment because measurements for the conductivity are involved. They allow scientists to determine whether water is clean and potable.
If the conductivity is high, this might be an indication of having too many dissolved things in the water, for example — salt or chemicals. They use that tool to ensure they the water is just right before it gets up through our taps.
Conductivity Test
The pharmaceutical companies are also using the solution conductance measurements for preparing medicines.
Drugs must be absolutely pure; the smallest amount of impurity can pose a problem. Using the measurement of conductivity, they can ensure that water used in pharmaceutical production is not contaminated with impurities.
This is led to ensure the safety and effectiveness of medicines.
We also see the use of conductivity measurements in food production. When preparing food, having the perfect ratio of condiments is also key.
So to them, say when they put some liquid ingredients for making a drink on the plate, it knows that how good this one mixes with electricity conductivity and decides if more or less amount of these ingredients are required. The taste or quality of the food might not be exactly as it should if we strike that balance wrong.
Thus, because of its myriad applications conductivity measurements are critical in maintaining the cleanliness or our water and safety of our medications has increasing prevalence humans consume medication that can interfere with electrolyte balance such as anti-diabetic drugs.
It is somewhat like a smart tool which ensures everything at the right place!
Conductivity in Weak and Strong Electrolytes
Electricity and its Behaviour in Various Solutions Lets Discuss about the electricity Judicially!! Some of you might probably know that there are miniscule particles called ions, so they carry electricity. In water, some substances dissociate into these ions to deliver electricity. This is conductivity for you.
Strong Electrolytes — Let’s say we have some fantastic sponge that absorbs water really (rapid particle movement).
A strong electrolyte is like the super sponge. Strong electrolytes break apart 100% when you put it in water (e.g. Table salt, NaCl)— this leads to ions.AbsoluteConstraints This is what makes it so good at conducting electricity. Ergo: Quite a good conductor, all right?
Weak Electrolytes: Think of a sponge but the sponge that is not very good at getting dry.
A sponge is a weak-electrolyte. When you dissolve a weak electrolyte, such as acetic acid (vinegar), in water, it does not fully dissociate. Instead, very few of the molecules become ions and majority stay largely as a single entity.
This means the solution doesn’t conduct electricity as well as a strong electrolyte.
Simply, strong electrolytes give rise to solutions that are very effective at conducting electricity because they produce many ions.
Solutions from weak electrolytes are poor at doing this as they form fewer ions. This helps us to understand how various substances can influence the flow of electricity in different situations!
Compare the Behavior of Weak and Strong Electrolytes With Respect to Conductivity Changes as Concentration Varies.
Now, suppose you had two beverages: lemonade and orange juice. Think of lemonade as a weak electrolyte, orange juice to be strong. They both can conduct electricity, but they do so in different ways which is highly dependent of the concentration added.
Strong electrolytes
Strong electrolytes are like orange juice. When you put orange juice in water, it gets stronger and stronger. Strong electrolytes, such as table salt, which is also known as Sodium Chloride or NaCl, break into ions entirely when they dissolve in water just like that.
These ions are like little electric charge carriers. And the more salt you have in that water, because this is all ions makes it easier for the electrons to move through and conducts electricity better.
This can be good, and the more salt you add to themotion attracts anions in’C’); This is bad (the motion we like attacted very much.
Weak Electrolytes
Weak electrolytes are like lemonade. Adding lemonade to water merely makes the solution only so slightly stronger or concentrated.
Sodium and Chlorine ions dissociated in water to form Na+ + Cl-(aq) Strong Electrolytes Weak electrolyte like vinegar will partially break into Ions. There are fewer ions than in the strong electrolyte solutions. Which means, however more lemonade you add, do not expect too many ions.
That is, spu:ng solutions are much less conductive than equivalent concentrations of strong electrolytes.
So overall, the greater you add strong ions like H2O are better conductors of power but not so much for weak ions and continuing to additional spoiler! Orange juice is like cooking concentrate where the higher it gets, stronger and more intense when being mix but lemonade never changes throughout the process.
Kohlrausch’s Law of Independent Migration of Ions
According to Kohlrausch’s Law of Independent Migration, ions (such as sodium or chlorine) freely move within a solution regardless if other ions are present.
Visualize that you are swimming with your friend in a pool-wave, and still if he swims faster than your speed will not increase. In the same way, every ion in a solution moves by itself.
This law helps scientists understand how different substances dissolve and conduct electricity in solutions.
Explain Kohlrausch’s Law and How it Relates to the Molar Conductivity of Electrolytes at Infinite Dilution
Well Kohlrausch’s law is kind of a rule for how well salts, and acids and bases conduct electricity when you dissolve them in water.
Take, for instance, your glass of salt water. The salt is divided into even smaller parts known as ions that are capable of carrying the electric current devoid from the water.
This shows us that we change the solution’s ability to conduct electricity if you keep adding water, and salt. When we add more and more water, the salt ions become farther apart from each other since they are being separated by some distance. … yep it really will not carry electrons to well any longer!
Which is a nice point to ponder about called the “infinite dilution”. This is when we add so much water that the salt is spread out as much as possible.
This is were Kohlrausch’s Law comes into the play and gives us an insight of the salt to conduct electricity at its best – i.e., molar conductivity at infinite dilution.
That’s how well the salt conducts electricity when it at its “diluted” best in water. It would be equivalent in performance to learning a race cars Top speed on an infinite track!
Ionic Mobility and Its Role in Conductivity
If you are at a party with friends, and everyone is rushing throughout the room in one order. Everyone quickly move to ones means a lively and exuberant party. 5 minutes is really wonderful wow it ended… )
But when folks are moving at a slow clip, the party is not quite as enjoyable. This is kinda like how ionic mobility works in electricity!
Ionic mobility is the rate of migration of ions (small charged particles) through a material. Conductivity is a measure of how well electric current can flow through material. While a party needs guests to be up and moving, materials need free-moving ions to conduct electricity.
Ions are free to move easily in materials like saltwater, making it a good conductor of electricity. Meanwhile materials such as rubber are bad at allowing ions to move so do not conduct electricity much.
This basically means the more easily positively and negatively charged ions can flow in a material, the better it is at conducting electricity. And… that is why ionic mobility helps to explain conduction of electricity in things!
Relationship Between Molar Conductivity and Concentration for Weak Electrolytes
It is like a big bowl of soup and you just want to taste how salty it is. With a little salt, it makes mildly salty soup. However, fill it with a splash more and the whole pot suddenly becomes much saltier. This is kinda like how weak electrolytes function in a solution.
What are Weak electrolytes?
These are substances that don’t dissolve in water—that is to say, they only partially break down into small particles known as ions. Split only slightly apart.
For example, when you dilute a weak acid such as vinegar in water, it is not completely divided into ions—some molecules will still hang together.
OK, so What is Molar Conductivity?
Molar conductivity indicates how well a solution can conduct electricity. This depends on the number of ions present in the solution.
Higher the ions, greater is its conductivity. When you dissolve a weak electrolyte in water, such as HF or H₂C₆H₅O₇, only very few ions are produced and this impacts how well the solution can conduct electricity.
The Variation of Conductivity and Molar Conductivity with Concentration should keep increasing as you add more of the weak electrolyte to the solution, right? Counterintuitively, the molar conductivity actually decreases as you increase the concentration of a weak electrolyte.
This is because at higher concentration, the ions in the solution are more crowded by each other, causing what we can consider a head-to-head hit.
The more bumpy it gets, the bigger the struggle ions have when trying to move around freely, and consequently, this reduces how well the solution can conduct electricity.
Ultimately, in the case of weak electrolytes—which are a type of ion compound that hardly has an attraction or repulsion effect on other ions—as more and more portions of these compounds’ specific particles establish contact with water molecules, they prevent it from exchanging free carriers of electrical current.
Well, I attempted to create a trance loop that was loaded with so much salt it disqualified itself from being more salty—it couldn’t taste the ocean.
Measuring Conductivity: Techniques and Instruments
One thing to pay attention when measuring conductivity is the ease with which electricity can flow through a particular substance.
So, lets think about electricity like water flowing through a pipe. If the pipe is wide, water can run through it. Then, when you have an area that is narrow or blocked, i.e., the water cannot easily pass through it. How easily electricity flows in materials (from very low to extremely high) is called conductivity.
Measuring Conductivity There are several techniques and instruments that can be used to measure conductivity. Conductivity meter- This is another common tool.
The device that this group built is sort of like a thermometer for electrons — it does not measure temperature, but how easily electricity flows through something. The probes of the meter are to be placed into whatever you’re testing, provides a reading.
Conductivity Probe – For Liquids An example often used is a probe-type temperature sensor… not directly in the liquid but instead one that you dip into it.
Which contains electrodes that measure how well the liquid conducts electricity. This is useful for activities like measuring the purity of water or grading various beverages.
For solids, resistance testers are commonly employed. Solid dielectric testers test the resistance of a solid to electrical flow. Less resistance = More better conductivity. They are also very useful testers for testing electrical cables and other materials.
Knowing how well a material can conduct electricity allows scientists, engineers, and even everyday people to learn more about said materials and their properties when they are placed in certain environments.
It is like having a super capacity, as if you knew the secret of what everything around was made out of.
Understanding the Limiting Molar Conductivity
Molar conductivity can be limiting: When we consider limit molar Conductivity, here we are concern when equilibrium of may not able to carry electricity at there highest state concentration.
Let’s say you have a huge bowl of soup. Thicker, more full tastes are present when soup is highly concentrated. When water was added in abundance on the other hand, it turned out watery and tasteless. This is true of solutions in science also.
To understand, in chemistry,’when we dilute a solution by adding e.g., water (or another solvent), the particles are spreading out more because they take up space between them’.
It makes a difference in how the solution can conduct electricity. The change in conductivity and molar conductivity with concentration gives us a clear idea of this.
Conductivity is relevant to how well electricity can flow through the solution. The molar conductivity of a substance reflects how good one mole in that particular unit conducts electricity.
Solutions generally exhibit reduced electrical conductivity when diluted.
However, somewhere out there is a particular spot — described as the limiting molar conductivity point by scientist Michael Lichtenberg — that if we dilute our solution even further than this essential point, it levels off — whoa-up!
This occurs because the particles are extremely far apart to do much interaction with one another.
Therefore, variation of conductivity and molar conductivity with concentration gives us an idea about how electro-conductive nature of a solution will vary when we start adding more water to it.
The reason we care about limiting molar conductivity is it gives scientists a sense of how well the substance can conduct electricity molecule for molecule when diluted very, very far.
The Role of Conductivity in Environmental Monitoring
Conductivity teaches us about how effective a liquid– such as water –can be in transporting electrical energy. The conductivity tells us a lot about the quality of the water.
This property is better known as conductivity, and in water it acts as a sort of superpower that shows us how much stuff like salts or minerals are dissolved into the liquid phase.
And if there are too many of these things, the water can be toxic to even plants and animals. That is why it becomes necessary to know how conductivity and molar conductivity varies with concentration.
It tells us how the capacity of water to conduct electricity behaves when various other substances are dissolved in it Molar conductivity.
Here, if you add more salt in a glass of water then the conductivity of that liquid will increase because it helps electricity to flow better.
Variation in conductivity and molar conductivity with concentration with the variations which are monitored by the scientists to access quality of water supplied through rivers, ponds or oceans. They use various measurements to check for salinity and pollutants in the water.
Conductivity is used commonly in environmental monitoring to sense changes in water quality due either to rainwater dilution of carbon dioxide, copper decay by-products etc., or contamination. A sudden spike in conductivity can also indicate contamination entering the water.
These changes in background levels occur so-to-speak behind closed doors and may not be visible to the naked eye save for observation at a smaller scale, but regular surveillance helps scientists determine when early intervention measures may need to be taken by us all as citizens of our world.
How conductivity is used to monitor pollution and water quality in environmental studies
You can simply think of conductivity as a test to see how good water is in conducting electricity and people may use this method (conductivity), scientists too, just to determine whether theses clean or polluted. A typically occurs conductivity increases as pollution rises.
This is the value that actually informs them how clean or dirty this water has become. They also learn the changes of conductivity with concentration (Variation of Conductivity and Molar Conductivity with Concentration) due to the presence of a variable amount/set quantity level by taking this from that.
Which helps them protect our drinking and recreational water. So essentially, conductivity is like our handy detective tool that helps protect the cleanliness and health of our water!
Comparison of Electrolytic and Nonelectrolytic Conductivity
Electrolytic and nonelectrolytic conductivity is a term used when we discuss the electrical conduction of any substance.
Substances, such as saltwater or lemon juice for example produce Electrolytic Conductivity. These materials are made up of ions, which are small particles that have an electric charge and can move around.
If you dissolve salt in water, it breaks down into sodium and chloride ions — allowing the water to conduct electricity. The ions move very freely and allow the passage of electrical current.
Literally nonelectrolytic the legitimacy in case of sugar mixed with water. That sugar molecules do not ionize here. They do not dissociate into ions; they dissolve as complete molecules. With no ions to travel through the solution, sugar solutions do not conduct electricity easily.
Conductometric measurements Conductivity and molar conductivity Variation of conductivity smoke molar conductance with concentration.
When more salt is added to water, it makes the solution carry current well as there are many ions in it. A saturated solution is a solution where you have added in too much solid for the amount of liquid and so when more powder will no longer dissolve into your liquid.
Thus, there is a great variability with the concentration of electrolytic solutions rather than non-electrolyte ones.
Key Takeaways on the Variation of Conductivity and Molar Conductivity with Concentration
Conclusively, ending with what we learned about the variation of conductivity and molar conductivity with concentration.
But when I mentioned variation of conductivity or molar conductivity with concentration, we are examining how these values change as the amount (concentration) of same substance is increased in a solution.
Conductivity: indicates the ability of a solution to conduct an electric current. We normally know, the higher we increase in any substance level so that will maintain at its maximum because more amount of particles are there to carry the current.
But when the particles are packed in at very high concentrations, or crowding about that tiny sidewall opening (0.1 x 2 mm), they behave quite differently and their trend may even reverse into jamming!
In molar conductivity, in contrast to specific conductance, an individual generating one mole contributes towards the general conductivit.
However, the molar conductivity usually decreases with increasing concentration since, at high concentrations ions become crowded in a way that you start to get solute-solute interactions (inelastic collisions).
Conclusion
What we have observed from the variation of conductivity and molar conductivity with concentration are – 1) Conductivity → Generally change with Concentration. 2) Molar conductance→ Change mode is different in both cases (direct/inverse).
Knowing this ‘ variation of conductivity and molar conductivity with concentration’ makes us understand more about the solution in different conditions.
To summarize the ‘Variation of Conductivity and Molar Conductivity with Concentration’ teaches us what is behind conducting electricity for substances.
Because both the conductivity and molar conductivities change as a solution with variable concentration is measured. Which for instance would make conductivity worse if you add more of that substance, but not a rule.
I hope this article will help you understand the concept of Variation Of Conductivity And Molar Conductivity With Concentration and make Chemistry learning an enjoyable experience.
Share with your family and friends.
